PHYSICAL SCIENCES: CHEMISTRY P1 Past Paper FEBRUARY/MARCH 2016 - GRADE 12 NATIONAL SENIOR CERTIFICATE
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY (P2)
FEBRUARY/MARCH 2016
MARKS: 150
TIME: 3 hours
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces in the ANSWER BOOK and on the GRAPH SHEET.
- This question paper consists of TEN questions. Answer QUESTION 5.3.2 on the attached GRAPH SHEET. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions. Each question has only ONE correct answer. Choose the answer and write only the letter (A–D) next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 Which ONE of the following compounds is an aldehyde?
- CH3COCH3
- CH3CH2CHO
- CH3CH2COOH
- CH3CH2CH2OH (2)
1.2 The equation below represents the decomposition of calcium carbonate.
CaCO3(s) -> CaO(s) + CO2(g)
Which ONE of the following factors will increase the initial rate of decomposition of calcium carbonate?
- Pressure
- Temperature
- Concentration
- Mass of CaCO3(s) (2)
1.3 Consider the cell notation of the galvanic cell below.
Zn│Zn2+║Cu2+│Cu
Which ONE of the following statements regarding this cell is TRUE?
- Copper is formed at the cathode.
- Copper is formed at the anode.
- Zinc is formed at the anode.
- Zinc is formed at the cathode. (2)
1.4
Which ONE of the following compounds will react with sodium hydroxide (NaOH) in a neutralisation reaction?
- CH3CHO
- CH3COOH
- CH3COCH3
- CH3CH2OH (2)
1.5 Consider the reactant Y in the following reaction:
Y + H2O ⇌ H3O+ + H2PO4-
The formula of Y is:
- PO43-
- H2PO4-
- HPO42-
- H3PO4 (2)
1.6
A gardener needs a fertiliser with the highest percentage of the relevant nutrient to obtain a green lawn.
Which ONE of the following NPK fertilisers will give the best results?
- 8 : 1 : 5
- 7 : 1 : 1
- 3 : 2 : 3
- 3 : 1 : 5 (2)
1.7
The activation energy for a certain reaction is 50 kJ∙mol-1. Energy is absorbed when this reaction takes place.
Which ONE of the following is CORRECT for the REVERSE reaction?
| ACTIVATION ENERGY (EA) | HEAT OF REACTION (ΔH) | |
| A | EA > 50 kJ∙mol-1 | ΔH > 0 |
| B | EA > 50 kJ∙mol-1 | ΔH < 0 |
| C | EA > 50 kJ∙mol-1 | ΔH < 0 |
| D | EA > 50 kJ∙mol-1 | ΔH > 0 |
1.8
Which ONE of the following pairs of compounds are FUNCTIONAL isomers?
- Methanol and methanal
- Butane and 2-methylpropane
- Propan-1-ol and propan-2-ol
- Propanoic acid and methyl ethanoate (2)
1.9
The balanced equations for three reactions at equilibrium in a closed container are given below.
- C2H4(g) + H2(g) ⇌ C2H6(g)
- Fe3O4(s) + 4H2(g) ⇌ 3Fe(s) + 4H2O(g)
- SO3(g) + NO(g) ⇌ NO2(g) + SO2(g)
In which reaction(s) will the equilibrium position shift when the volume of the reaction vessel is decreased at constant temperature?
- (i) only
- (i) and (ii) only
- (i) and (iii) only
- (i), (ii) and (iii) (2)
1.10
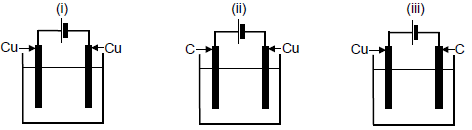
In each of the electrolytic cells below, copper(II) sulphate is used as the electrolyte. The electrodes are either carbon (C) or copper (Cu).
In which cell(s) will the concentration of the electrolyte remain constant during electrolysis?
- (i) only
- (i) and (ii) only
- (i) and (iii) only
- (ii) and (iii) only (2) [20]
QUESTION 2 (Start on a new page.)
2.1
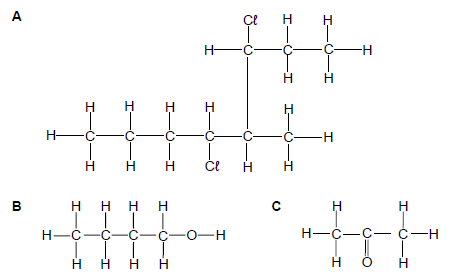
Consider the organic compounds represented by the letters A to C below.
Write down the:
2.1.1 Name of the homologous series to which compound C belongs (1)
2.1.2 IUPAC name of compound A (3)
2.1.3 Structural formula of a tertiary alcohol that is a structural isomer of compound B (2)
2.2
An alcohol and methanoic acid are heated in the presence of concentrated sulphuric acid to form an ester.
2.2.1
What is the role of the concentrated sulphuric acid in this reaction? (1)
2.2.2
Write down the NAME or FORMULA of the inorganic product formed.(1)
The ester contains 6,67% hydrogen (H), 40% carbon (C) and 53,33% oxygen (O). The molar mass of the ester is 60 g·mol-1.
Use a calculation to determine its:
2.2.3 Empirical formula (5)
2.2.4 Molecular formula (3)Write down the:
2.2.5 Structural formula of methanoic acid (1)
2.2.6 IUPAC name of the ester (2)
[19]
QUESTION 3 (Start on a new page.)
3.1 Define the term boiling point. (2)
3.2
What is the relationship between strength of intermolecular forces and boiling point? (1)
The relationship between strength of intermolecular forces and boiling point is investigated using four organic compounds from different homologous series. The compounds and their boiling points are given in the table below.
| COMPOUND | BOILING POINT (°C) | |
| A | Propane | -42 |
| B | Propan-2-one | 56 |
| C | Propan-1-ol | 97 |
| D | Propanoic acid | 141 |
3.3
Refer to the TYPE and the STRENGTH of intermolecular forces to explain the difference in boiling points between:
3.3.1 Compounds A and B (3)
3.3.2 Compounds C and D (3)
3.4
Is compound B a GAS or a LIQUID at room temperature? (1)
[10]
QUESTION 4 (Start on a new page.)
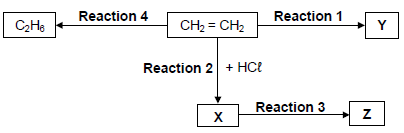
The flow diagram below shows different organic reactions using CH2 = CH2 as the starting reactant. X, Y and Z represent different organic compounds.
4.1
During Reaction 1, CH2 = CH2 undergoes polymerisation to form compound Y.
For this reaction, write down the:
4.1.1 Type of polymerisation (1)
4.1.2 NAME of compound Y (1)
4.2 For Reaction 2, write down the:
4.2.1 IUPAC name of compound X (2)
4.2.2 Type of addition reaction of which this is an example (1)
4.3 During Reaction 3, compound X reacts with excess hot water.
Write down the:
4.3.1 STRUCTURAL FORMULA of compound Z (2)
4.3.2 NAME or FORMULA of the INORGANIC product (1)
4.4 Reaction 4 is an addition reaction.
4.4.1
Is C2H6 a SATURATED or an UNSATURATED compound? Give a reason for the answer. (2)
4.4.2
Write down the NAME or FORMULA of the INORGANIC reactant needed for this reaction. (1)
4.4.3
Using molecular formulae, write down a balanced equation for the complete combustion of C2H6. (3)
[14]
QUESTION 5 (Start on a new page.)
NOTE: The graph for QUESTION 5.3.2 must be drawn on the GRAPH SHEET attached at the end of the QUESTION PAPER.
Methanol and hydrochloric acid react according to the following balanced equation:
CH3OH(aq) + HCl(aq) -> CH3Cl(aq) + H2O(l)
5.1 State TWO factors that can INCREASE the rate of this reaction. (2)
5.2 Define the term reaction rate. (2)
5.3
The rate of the reaction between methanol and hydrochloric acid is investigated. The concentration of HCl(aq) was measured at different time intervals. The following results were obtained:
| TIME (MINUTES) | HCl CONCENTRATION (mol∙dm-3) |
| 0 | 1,90 |
| 15 | 1,45 |
| 55 | 1,10 |
| 100 | 0,85 |
| 215 | 0,60 |
5.3.1
Calculate the average reaction rate, in (mol∙dm-3)∙min-1 during the first 15 minutes. (3)
5.3.2
Use the data in the table to draw a graph of concentration versus time on the attached GRAPH SHEET.
NOTE: The graph is not a straight line.
(ATTACH THIS GRAPH SHEET TO YOUR ANSWER BOOK.) (3)
5.3.3
From the graph, determine the concentration of HCℓ(aq) at the 40th minute. (1)
5.3.4
Use the collision theory to explain why the reaction rate decreases with time. Assume that the temperature remains constant. (3)
5.3.5
Calculate the mass of CH3Cl(aq) in the flask at the 215th minute. The volume of the reagents remains 60 cm3 during the reaction. (5)
[19]
QUESTION 6 (Start on a new page.)
Initially, 2,2 g of pure CO2(g) is sealed in an empty 5 dm3 container at 900 °C.
6.1 Calculate the initial concentration of CO2(g). (4)
6.2 Give a reason why equilibrium will not be established. (1)
CaCO3(s) is now added to the 2,2 g CO2(g) in the container and after a while equilibrium is established at 900 °C according to the following balanced equation:
CaCO3(s) ⇌ CaO(s) + CO2(g)
The equilibrium constant for this reaction at 900 °C is 0,0108.
6.3
Give a reason why this reaction will only reach equilibrium in a SEALED container. (1)
6.4
Calculate the minimum mass of CaCO3(s) that must be added to the container to achieve equilibrium. (7)
6.5
How will EACH of the following changes affect the amount of CO2(g)? Write down only INCREASES, DECREASES or REMAINS THE SAME.
6.5.1 More CaCO3(s) is added at 900 °C (1)
6.5.2 The pressure is increased (1)
6.6
It is found that the equilibrium constant (Kc) for this reaction is 2,6 x 10-6 at 727 °C. Is the reaction EXOTHERMIC or ENDOTHERMIC? Fully explain how you arrived at the answer. (4)
[19]
QUESTION 7 (Start on a new page.)
7.1 Define an acid in terms of the Lowry-Brønsted theory. (2)
7.2
Carbonated water is an aqueous solution of carbonic acid, H2CO3. H2CO3(aq) ionises in two steps when it dissolves in water.
7.2.1 Write down the FORMULA of the conjugate base of H2CO3(aq). (1)
7.2.2
Write down a balanced equation for the first step in the ionisation of carbonic acid. (3)
7.2.3
The pH of a carbonic acid solution at 25 °C is 3,4. Calculate the hydroxide ion concentration in the solution. (5)
7.3 X is a monoprotic acid.
7.3.1 State the meaning of the term monoprotic. (1)
7.3.2
A sample of acid X is titrated with a standard sodium hydroxide solution using a suitable indicator.
At the endpoint it is found that 25 cm3 of acid X is neutralised by 27,5 cm3 of the sodium hydroxide solution of concentration 0,1 mol∙dm-3.
Calculate the concentration of acid X. (5)
7.3.3
The concentration of H3O+ ions in the sample of acid X is 2,4 x 10-4 mol∙dm-3.
Is acid X a WEAK or a STRONG acid? Explain the answer by referring to the answer in QUESTION 7.3.2.(3)
[20]
QUESTION 8 (Start on a new page.)
An electrochemical cell consisting of half-cells A and B is assembled under standard conditions as shown below.
| Half-cell A | Pt, Cl2 (101,3 kPa) | Cl- (1 mol∙dm-3) |
| Half-cell B | Mg2+ (1 mol∙dm-3) | Mg(s) |
8.1 At which half-cell, A or B, are electrons released into the external circuit? (1)
8.2 Write down the:
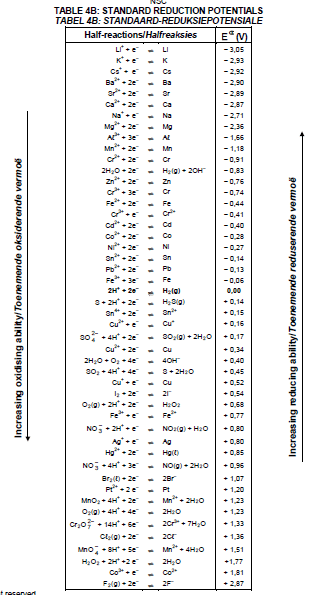
8.2.1 Reduction half-reaction that takes place in this cell (2)
8.2.2
NAME or FORMULA of the substance whose oxidation number DECREASES (1)
8.3 Calculate the initial cell potential of this cell when it is in operation. (4)
8.4
Write down an observation that will be made in half-cell B as the cell operates. Give a reason for the answer.(2)
[10]
QUESTION 9 (Start on a new page.)
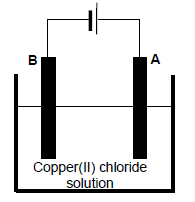
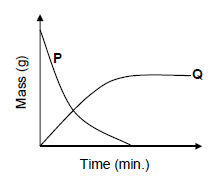
The electrochemical cell below is set up to demonstrate the purification of copper.
9.1 Write down the type of electrochemical cell illustrated above. (1)
The graphs below show the change in mass of the electrodes whilst the cell is in operation.
9.2 Define a reducing agent in terms of electron transfer. (2)
9.3 Which graph represents the change in mass of electrode A? (1)
9.4 Write down the half-reaction that takes place at electrode A. (2)
9.5
Electrodes A and B are now replaced by graphite electrodes. It is observed that chlorine gas (Cl2) is released at one of the electrodes.
At which electrode (A or B) is chlorine gas formed? Fully explain how it is formed.(3)
[9]
QUESTION 10 (Start on a new page.)
Ammonium nitrate is an important fertiliser. It is produced by reacting nitric acid with ammonia. Both nitric acid and ammonia are prepared on a large scale in industry.
10.1 Write down the name of the industrial preparation of nitric acid. (1)
10.2
The catalytic oxidation of ammonia is one of the steps in the process named in QUESTION 10.1.
Write down the NAMES or FORMULAE of the TWO products formed in this step. (2)
10.3 Write down a balanced equation for the preparation of ammonium nitrate. (3)
10.4
Calculate the mass, in kilogram, of ammonium nitrate that can be made from 6,8 x 104 kg of ammonia and excess nitric acid.
(One mole of ammonia produces one mole of ammonium nitrate.) (3)
10.5
Ammonium nitrate is often mixed with potassium chloride and ammonium phosphate. Give a reason why it is mixed with these compounds. (1)
[10]
TOTAL: 150