Matter and Materials Questions and Answers Grade 10
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupQuestions
Question 1: Multiple choice
Choose the correct answer. Only write the letter of the answer you select.
1.1 Where are metals found on the periodic table?
- At the bottom
- To the right
- To the left
- At the top (3)
1.2 What is the name given to the group VII elements?
- Alkali metals
- Alkaline earth metals
- Halogens
- Nobel gases (3)
1.3 What change to a neutral atom will result in the formation of a negative ion?
- It gains an electron.
- It gains a proton.
- It loses an electron.
- It loses a proton. (3)
1.4 Which statement about the numbers of particles in atoms is correct? Apart from hydrogen, most atoms contain:
- more neutrons than protons.
- more protons than neutrons.
- more electrons than protons.
- more protons than electrons. (3)
1.5 Metal atoms form:
- positive anions.
- negative anions.
- negative cations.
- positive cations. (3)
1.6 Which are the correct formulae for sodium chloride and calcium carbonate?
- NaCl and CaCO3
- SCl and CaCO3
- NaCl and CaCO2
- NaCl and CmCO3 (3)
1.7 Which of the elements below has an electron configuration of 1s22s22p4?
- sodium
- chlorine
- oxygen
- fluorine (3)
1.8 Which of the following terms describes the change in state that occurs when a liquid changes into a solid?
- condensation
- evaporation
- freezing
- sublimation (3)
1.9 Copper has two isotopes; 69,1% of copper isotopes have a mass of 63 and 30,9% have a mass of 65. What is the average mass of a copper atom?
- 65
- 66
- 64,4
- 63,6 (3)
1.10 In which of the following compounds are electrons shared between atoms?
- sodium fluoride
- nitrogen dioxide
- iron bromide
- 1 only
- 2 only
- 1 and 3
- 1, 2 and 3 (3)
1.11 Which compound contains two double bonds in which electrons have been shared?
- hydrogen bromide
- carbon dioxide
- sodium iodide
- water (3)
1.12 In the molecules CH4, HBr and H2O, which atoms use all of their outer shell electrons in bonding?
- C and Br
- C and H
- Br and H
- H and O (3)
1.13 The following statement is about chemical bonding. Covalent bonds are formed by the … of electrons. Covalent bonds occur between ... Which combination of words completes the statement? (3)
A | transfer | two non-metals |
B | transfer | a non-metal and a metal |
C | sharing | two metals |
D | sharing | two non-metals |
1.14 Which of the elements below is most likely to form a positive ion?
- zinc
- chlorine
- oxygen
- fluorine (3)
1.15 Which substance when combined with oxygen will form a covalent bond?
- sodium
- magnesium
- boron
- aluminium (3) [45]
Question 2: Matching pairs
Choose an item from column B that matches the description in column A. Write only the letter of your choice (A–J) next to the question number.
Column A | Column B |
2.1 formation of positive ions | A – the number of protons and neutrons added together |
2.2 mass number | B – loss of electrons |
2.3 group VIII elements | C – when a substance changes from a liquid to a solid state |
2.4 chromatography | D – the number of protons and electrons added together |
2.5 condensation | E – halogens |
F – gain of electrons | |
G – separation of mixtures of pigments/colours | |
H – when a substance changes from a gas to a liquid state | |
I – separation of a solid from a liquid | |
J – noble gases |
[5]
Question 3: True/false
Indicate whether the following statements are true or false. If the statement is false, write down the correct statement.
3.1 Isotopes are elements with the same number of protons, but different numbers of electrons. (2)
3.2 Isotopes contain the same number of electrons in their outermost energy shell. (2)
3.3 Sodium has an electron configuration of 1s22s22p63s1. (2)
3.4 Calcium and magnesium both form anions with a charge of +2. (2)
3.5 A mixture can be separated into its component substances by physical means. (2) [10]
Question 4: One-word answers
Provide one word or term for each of the descriptions. Write only the word or term next to the question number.
4.1 The movement of particles from a region where there are many to a region where there are fewer. (1)
4.2 The basic building block of matter. (1)
4.3 The electrons found in the outermost energy shell. (1)
4.4 A mixture in which the different substances that make up that mixture can be seen. (1)
4.5 The type of chemical bonding that occurs when electrons are transferred from one atom to another. (1) [5]
Question 5: Long questions
Mixtures can be homogeneous or heterogeneous.
5.1 What is the difference between a homogeneous mixture and a heterogeneous mixture? (2)
5.2 Give one example of each. (2)
5.3 How do mixtures differ from compounds? (2)
5.4 Which of the following substance is pure?
- sugar
- sea water
- steel (1) [7]
Question 6: Long questions
The following are the melting points of the metals in group II on the periodic table:
- beryllium 1278 °C
- magnesium 649 °C
- calcium 839 °C
- strontium 769 °C
- barium 725 °C
6.1 What is the general trend in melting points as you go down the group? (1)
6.2 One metal does not fit this trend. Which one is it? (1)
6.3 Does this information support the idea that beryllium atoms are held together more strongly than barium atoms? (1)
6.4 Explain your answer to question 6.3. (2) [5]
Question 7: Long questions
Use the table below to answer the questions that follow.
Substance | Melting point (°C) | Boiling point (°C) |
lead | 317 | 174 |
radon | –71 | –62 |
ethanol | –117 | 78 |
cobalt | 1492 | 2900 |
nitrogen | –210 | –196 |
propane | –188 | –42 |
ethanoic acid | 16 | 118 |
7.1 Define the boiling point of a substance. (2)
7.2 Which two substances are gaseous at –50 °C? (2)
7.3 Which substance is a liquid at 2500 °C? (1)
7.4 Is nitrogen a liquid, solid or gas at 35 °C? (1) [6]
Question 8: Long questions
The table below shows information about two isotopes of chlorine.
Atom | Number of protons | Number of electrons | Number of neutrons |
Chlorine-35 | A | 17 | 18 |
Chlorine-37 | 17 | B | C |
8.1 Replace the letters with the correct numbers to complete the table. (3)
8.2 Define isotopes. (2)
8.3 Draw an Aufbau diagram of a chlorine atom. (2)
8.4 A chlorine ion has a charge of –1. Write down the electron configuration for a chlorine ion. (1) [8]
Question 9: Long questions
The diagram below shows the electron configuration of four different elements. 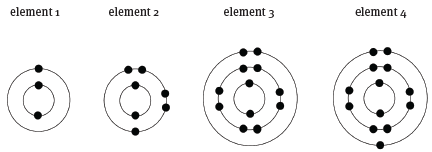
9.1 Which element has an atomic number of 3? (1)
9.2 Which atom has the electron configuration 1s22s2 2p63s2? (1)
9.3 Which element is nitrogen? (1) [3]
Question 10: Long questions
The structure of a typical ionic compound is a regular arrangement of positive and negative ions.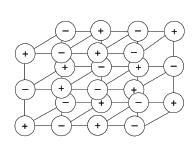
10.1 What is the name of this regular arrangement of particles? (1)
10.2 Name an ionic substance. (1)
10.3 Ions are formed by electron loss or gain.
10.3.1 Give the formula of the magnesium ion. (1)
10.3.2 Give the formula of the oxide (oxygen) ion. (1)
10.3.3 Why are these two ions attracted to each other? (1)
10.3.4 Draw a Lewis diagram to show the bonding that takes place between a magnesium and oxygen atom. (3) [8]
Question 11: Long questions
The table below describes the number of protons, neutrons and electrons found in three different substances: A, B and C.
Particle | Number of protons | Number of electrons | Number of neutrons |
A | 15 | 15 | 16 |
B | 15 | 18 | 16 |
C | 15 | 15 | 17 |
Use the information in the table to explain why the following statements are true.
11.1 Particle A is a neutral atom. (1)
11.2 They are all particles of the same element. (1)
11.3 Particle B is a negative ion. (1)
11.4 Particles A and C are isotopes. (2)
11.5 What is the charge on particle B? (1)
11.6 Is particle B a metal or a non-metal? Give a reason for your answer. (2) [8]
Answers to questions
Question 1: Multiple choice
1.1 C
1.2 C
1.3 A
1.4 B
1.5 D
1.6 A
1.7 C
1.8 C
1.9 D
1.10 B
1.11 B
1.12 B
1.13 D
1.14 A
1.15 C [45]
Question 2: Matching Pairs
2.1 B
2.2 A
2.3 J
2.4 G
2.5 H [5]
Question 3: True/False
3.1 False – Isotopes have the same number of protons, but different numbers of neutrons.
3.2 True
3.3 True
3.4 False – Sodium and calcium both form cations with a charge of +2.
3.5 True [10]
Question 4: One-word answers
4.1 diffusion
4.2 atom
4.3 valence electrons
4.4 heterogeneous
4.5 ionic [5]
Question 5: Long questions
5.1 In a homogeneous mixture, the substances that make up the mixture are not visible as separate substances whereas in a heterogeneous mixture they are.
5.2 Homogeneous – sea water, fruit juice, tea, coffee, steel (any correct one)
Heterogeneous – granite, nuts and raisins, smoke, oil and water, box of biscuits (any correct one)
5.3 In mixtures, the substances that make up that mixture can be in variable proportions. In compounds, the elements combine in fixed ratios. Mixtures can be separated by physical means, while compounds can only be separated by chemical means.
5.4 Only sugar is a compound. The rest are mixtures. [7]
Question 6: Long questions
6.1 melting points decrease
6.2 magnesium
6.3 yes
6.4 because the melting point of beryllium is much higher, so more energy is needed to melt it [5]
Question 7: Long questions
7.1 The boiling point of a substance is the temperature at which that substance changes from a liquid into a gas.
7.2 radon and nitrogen
7.3 cobalt
7.4 gas [6]
Question 8: Long questions
8.1 A 17
B 17
C 20
8.2 Elements with the same atomic number, but different mass numbers.
8.3
- correct number of electrons
● correct placement of electrons
8.4 1s22s22p63s23p6 [8]
Question 9: Long questions
9.1 element 1
9.2 element 3
9.3 element 2 [3]
Question 10: Long questions
10.1 crystal lattice
10.2 Any metal atom combined with a non-metal atom.
10.3 10.3.1 Mg+2
10.3.2 O–2
10.3.3 The electrostatic attraction between oppositely charged ions.
10.3.4
- correct number of valence electrons
- correct placement of valence electrons
- correct charges of ions [8]
Question 11: Long questions
11.1 It has the same number of protons as electrons.
11.2 They all have the same number of protons.
11.3 It has more electrons than protons.
11.4 They have the same number of protons, but different numbers of neutrons.
11.5 –3
11.6 It is a non-metal, as it has formed a negative ion. [8]