OPTICAL PHENOMENA AND PROPERTIES OF MATERIALS QUESTIONS AND ANSWERS
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupWorked example 1
Calculate the energy of a light wave with a wavelength of 660 nm.
Solutions
c = λf
∴ 3 × 108 = 660 × 10–9 f
f = 3 × 108 – 4,55 × 1014 Hz
E = hf
E = (6,63 × 10–34)(4,55 × 1014)
E = 3,02 × 10–19 J
When the wavelength is given, always calculate the frequency first.
Convert nm to m 660 nm = 660 × 10–9 m
Use c = λf
Then calculate the energy using E = hf.
Worked example 2
A learner wants to demonstrate the photoelectric effect.
He uses a disk of zinc placed on an electroscope.
The work function (W0) of zinc is 6,9 × 10–19 J.
- Define the concept work function.
- Calculate the maximum wavelength of light that will eject electrons from the zinc.
- The electroscope is negatively charged and then exposed to ultraviolet light from a mercury discharge lamp. One of the wavelengths of the light is 260 nm. Calculate the kinetic energy of an electron emitted from the zinc disk by a photon of this light.
Solutions
- The work function (W0) of a metal is the minimum energy that is required to emit a photoelectron form the surface of the metal.
- E = hf and c = λf
6,9 × 10–19 = (6,63 × 10–34) f 3 × 108 = λ (1,05 × 1015)
f = 1,04 × 1015 Hz λ = 2,88 × 10–7 m - E = W0 + Ek
E = hf
hf = W0+ Ek
(6,63 × 10–19)(1,15 × 1015) = 6,9 × 10–19 + Ek
Ek = (7,63 × 10–19) – (6,9 × 10–19)
Ek = 7,3 × 10–20 J
And
c = λf
3 × 108 = (260 × 10–9) f
f = 3 × 108 / (260 × 10–9)
f = 1,15 × 1015 Hz
Remember: Convert nm to m 260 nm = 260 × 10–9 m
Activity 1
A metal surface is illuminated with ultraviolet light of wavelength 330 nm. Electrons are emitted from the metal surface. The minimum amount of energy required to emit an electron from the surface of this metal is 3,5 × 10–19 J.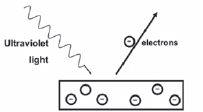
- Name the phenomenon illustrated. (1)
- Give ONE word or term for the underlined sentence in the above paragraph. (1)
- Calculate the frequency of the ultraviolet light. (3)
- Calculate the kinetic energy of a photoelectron emitted from the surface of the metal when the ultraviolet light shines on it. (3)
- The intensity of the ultraviolet light illuminating the metal is now increased.
What effect will this change have on the following?- Kinetic energy of the emitted photoelectrons. (Write down only INCREASES, DECREASES or REMAINS THE SAME.) (1)
- Number of photoelectrons emitted per second. (Write down only INCREASES, DECREASES or REMAINS THE SAME.) (1)
[10]
Solutions
|
Activity 2
In the simplified diagram below, light is incident on the emitter of a photocell. The emitted photoelectrons move towards the collector and the ammeter registers a reading.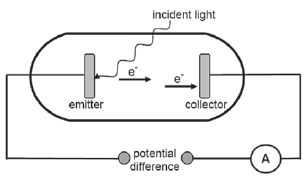
- Name the phenomenon illustrated above. (1)
- The work function of the metal used as emitter is 8,0 × 10–19 J. The incident light has a wavelength of 200 nm. Calculate the maximum speed at which an electron can be emitted. (6)
- Incident light of a higher frequency is now used.
How will this change affect the maximum kinetic energy of the electron emitted in the question above?
Write down only INCREASES, DECREASES or REMAINS THE SAME. (1) - The intensity of the incident light is now increased.
How will this change affect the speed of the electron calculated in QUESTION 11.1.2? Write down INCREASES, DECREASES or REMAINS THE SAME. Give a reason for the answer. (2) - A metal worker places two iron rods, A and B, in a furnace.
After a while he observes that A glows deep red while B glows orange. Which rod A or B has higher energy of radiation?
Give a reason for your answer. (2) - Neon signs illuminate many buildings.
What type of spectrum is produced by neon signs? (1)
[13]
Solutions
|
Activity 3
During an investigation, light of different frequencies is shone onto the metal cathode of a photocell. The kinetic energy of the emitted photoelectrons is measured. The graph below shows the results obtained.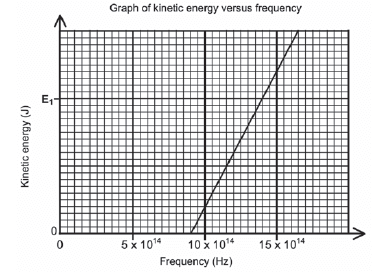
- For this investigation, write down the following:
- Dependent variable (1)
- Independent variable (1)
- Controlled variable (1)
- Define the term threshold frequency. (2)
- Use the graph to obtain the threshold frequency of the metal used as cathode in the photocell. (1)
- Calculate the kinetic energy at E1 shown on the graph. (4)
- How would the kinetic energy calculated in QUESTION 11.4 be affected if light of higher intensity is used? Write down only INCREASES, DECREASES or REMAINS THE SAME. (1)
[11]
Solutions
|