ORGANIC COMPOUNDS AND MACROMOLECULES GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
Share via Whatsapp Join our WhatsApp Group Join our Telegram Group- Organic compounds
- Physical properties and structure
- Physical properties of organic compounds
- Factors that have an influence on the physical properties of organic compounds
- Solids, liquids and gases
- Chemical properties of organic compounds
- Reactions of organic compounds
- Reactions of different homologous series
- Creating one hydrocarbon from another
- Plastics and polymers
- Plastics and pollution
Organic compounds and macromolecules
Organic chemistry is the chemistry of carbon compounds in living and non-living systems.
Organic molecules: molecules containing carbon atoms
Note: Carbon dioxide (CO2), carbon monoxide (CO), carbonates (CO32–) and cyanides (CN–) are exceptions because they are considered inorganic compounds. Organic compounds typically have a backbone of linked carbon atoms that other atoms attach to.
1.1 Organic compounds
1.1.1 Chemical bonding
The organic compounds that we study consist of Carbon, Hydrogen, Oxygen atoms and the Halogens ( -Br, -Cl, -I ), which are often represented collectively as –X).

1.1.2 The homologous series
DEFINITIONS
A homologous series is a series of compounds (molecules) that has the same general formula and the same functional group. Each member of a homologous series differs from the previous member by a – CH2 group.
Saturated: compounds in which there are no multiple bonds between carbon atoms in their hydrocarbon chains
Unsaturated: compounds with one or more multiple bonds between carbon atoms in their hydrocarbon chains.
A functional group is a bond, an atom or a group of atoms that determine(s) the physical and chemical properties of a group of organic compounds.
Classification of organic molecules according to homologous series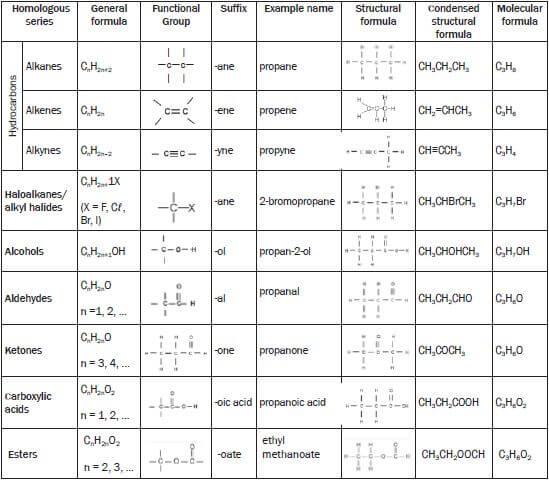
Note:
Alkanes have the general formula CnH2n+2
In propane n = 3 and therefore the general formula is C3H2(3)+2 = C3H8
In a structural formula all the bonds in the compound are shown. If any of these bonds are omitted, it is called a condensed structural formula.
E.g. structural formula for propane possible condensed structural formula for propane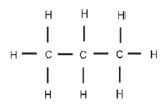 CH3 - CH2 - CH3 or CH3CH2CH3
CH3 - CH2 - CH3 or CH3CH2CH3
1.1.3 Isomers
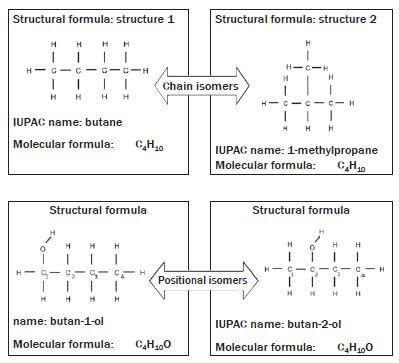
In isomers, the same atoms are used in different compounds, but they are assembled or connected in different ways. There are different kinds of isomers in organic chemistry. Structural isomers refer to the order in which different atoms in a compound are combined.
We will focus on three types of structural isomers, namely chain isomers, positional isomers and functional isomers.
NB
DEFINITIONS
Structural isomers are compounds with the same molecular formulae, but different structural formulae.
Chain isomers: Molecules with the same molecular formula and in the same homologous series but different types of chains e.g. butane and methyl propane.
Positional isomers: Molecules with the same molecular formula and in the same homologous series but the position of the functional group differs (e.g. butan-1-ol and butan-2-ol) or the substituent or side chains differ.
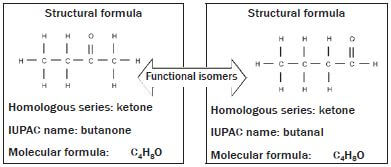
Functional isomers: Molecules with the same molecular formula but in different homologous series or have different functional groups e.g. ketones and aldehydes.
NB
The numbering of the carbon chain:
- In the naming of the organic compound, the longest carbon chain is numbered in such a way that the –OH functional group is attached to the carbon that is assigned the smallest number. Therefore, in this example, the first organic compound is called butan-1-ol.
In this example both organic compounds consist of the same number of carbon, hydrogen and oxygen atoms, but in the structure of the first compound, the (=) is attached to the middle of the carbon chain, which makes it a ketone. In the second example, the (=O) is attached to the beginning of the carbon chain, which makes it an aldehyde.
Activity 1
For each of the following isomers:
Write down the structural formula
Identify the type of isomer
1. 2-methylpentane and 3-methylpentane.
2. 1-chlorobutane and 1-chloro-2-methylpropane
3. Propanoic acid and ethyl methanoate (13) [13]
Solutions:
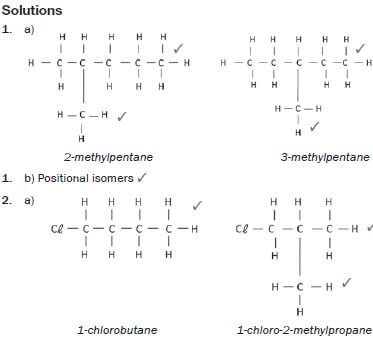
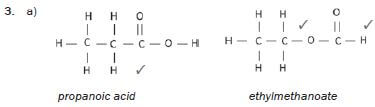
hint
Structural isomers have different IUPAC names and different physical properties.
1.1.4 Nomenclature (naming conventions or rules)
Each IUPAC name consists of three parts:
Prefix: | Root: | Suffix: |
| Position and names of substituents (side chains), listed alphabetically. | Number of C-atoms in the main C-chain. | Determined by the homologous series. |
Organic compounds are named according to the IUPAC (International Union of Pure and Applied Chemistry) system.
General steps for the IUPAC naming of all organic compounds
Step 1:
Identify the functional group in the compound and the homologous series it belongs to. This determines the suffix (ending). Example: Naming of 2-methylprop-1-ene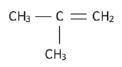
In this case there are only carbons and hydrogens, with a double bond between the carbons. This indicates that this compound is an alkene and will end with the suffix -ene
Step 2:
- Find the longest chain of carbon atoms. It must include the functional group, and need not be in a straight line.
- Number the carbon atoms in this chain from the side nearest to the functional groupg. a double or triple bond, a hydroxyl group, a carbonyl group or a carboxyl group.
- In the case of alkanes or haloalkanes, start numbering from the carbon nearest to a substituent (side chain)g. an alkyl group or halogen atom.
NB
Determine the root of the name from the number of C-atoms in the longest (main) C-chain.
- 1 = meth-
- 2 = eth-
- 3 = prop-
- 4 = but-
- 5 = pent-
- 6 = hex-
- 7 = hept-
- 8 = oct-
- 9 = non-
Note: old names: 1 = form- ; 2 = acet-
- Indicate the position of the functional group (except in the case of the alkanes). For alkenes and alkynes, give the smaller of the numbers of the C-atoms between which the double or triple bond exists.
Example: Naming of 2-methylprop-1-ene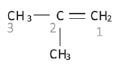
Example: The longest chain that includes the functional group, is numbered in the example above. Since the double bond is to the right of this example, we number the longest chain from right to left. The longest chain with the functional group contains three carbons and we know it must be a propene. Since the double bond is attached to the first carbon, it must be a prop-1-ene.
Step 3 :
- Determine whether there are any substituents.
- Count the number of C-atoms in substituent to determine the prefix and end it on -yl.
- Write the number of the C-atom on the main chain where the alkyl group is attached, in front of the alkyl name.
- Separate the number and the name with a hyphen, e.g. 2-methyl.
- If a substituent occurs more than once, use the appropriate prefix: twice - di; three times - tri; four times - tetra eg. 3,4-dimethyl.
- If there is more than one substituent, write their names and positions in alphabetical order.
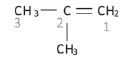
Example: The methyl group is attached to the second carbon, hence the name 2-methyl prop-1-ene
Step 4:
- Naming the organic compound with substituent(s) in front of the parent chain. Example: the IUPAC name of the compound discussed above: 2-methylprop-1-ene
Worked example 1
Give the IUPAC name for the following organic compound:
Step 1: Identify the functional group, and the homologous series to which this compound belongs. There are only C-atoms and H-atoms present, so this is a hydrocarbon. There are only single bonds between the C-atoms, therefore this is an alkane and the suffix will be –ane.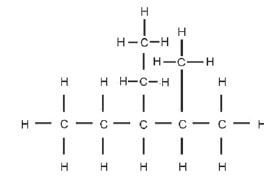
Step 2: Find the longest C-chain and count the C-atoms in it, starting at the side closest to the functional group. This is an alkane, so we start numbering from the side closest to a substituent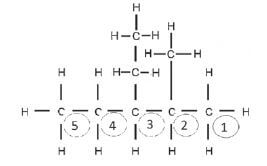
Step 3: Look at the substituents. Count the C-atoms in each substituent, determine the prefix and end it on –yl. The alkyl groups must be listed alphabetically, so we write the ethyl before the methyl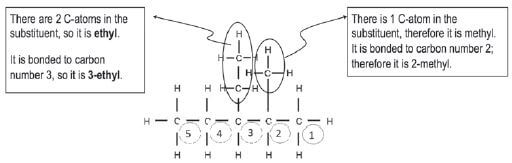
Hint:
- Numbers and letters of the alphabet are separated by a hyphen.(e.g 2-methylpropane)
- Numbers are separated by the comma, and no space between the substituent(s) and the parent chain. ( e.g. 2,2-dichloropentane)
e.g. Worked example 2
Give the IUPAC-name of the following haloalkane: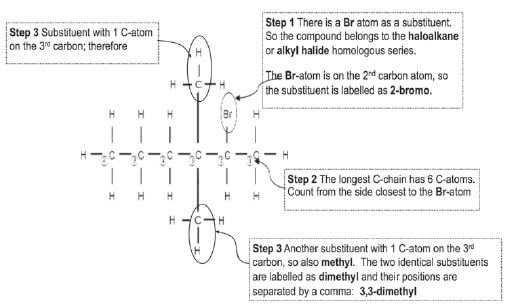
e.g. Worked example 3
Give the IUPAC-name of the following alkene: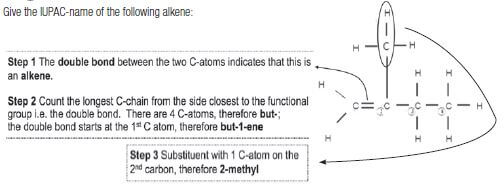
Solution: The compound is therefore 2-methylbut-1-ene.
e.g. Worked example 4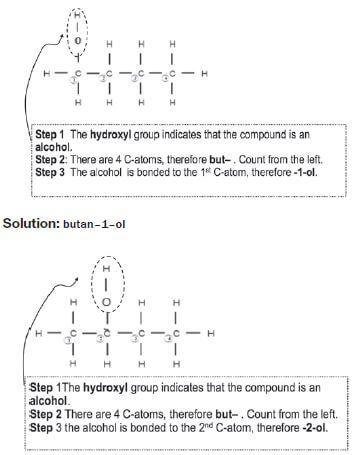
Solution:: butan–2–ol
e.g. Worked example 5
Give the IUPAC name for the following carboxylic acids: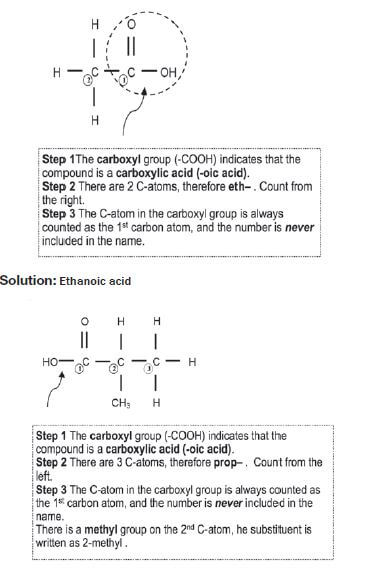
Solution: 2–methylpropanoic acid
e.g. Worked example 6
Give the IUPAC name for the following aldehyde: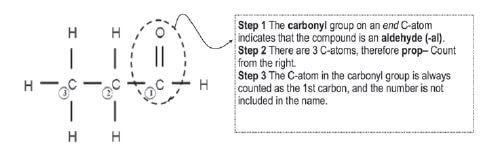
Solution: propanal
e.g. Worked example 7
Give the IUPAC name for the following ketone 
Solution: pentan–2–one
e.g. Worked example 8
Give the IUPAC name for the following ester: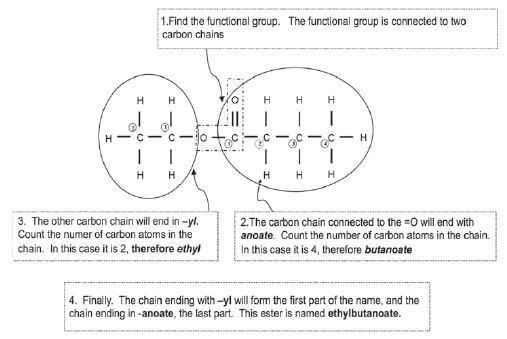
Activity 2
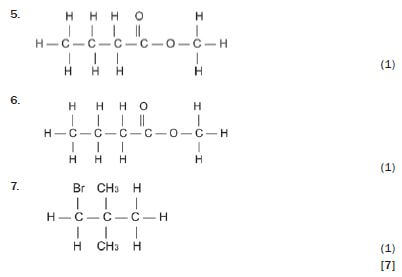
Write down the IUPAC names for the following organic molecules.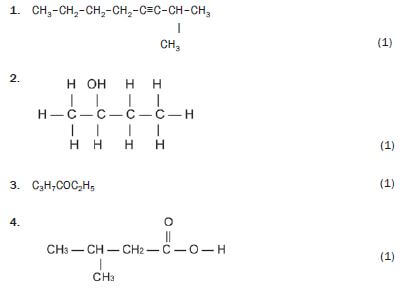
Solutions
1. 2-methyloct-3-yne 3 (1)
2. butan-2-ol 3 (1)
3. butanal 3 (1)
4. hexan-3-one 3 (1)
5. 3-methyl-butanoic acid 3 (1)
6. methyl butanoate 3 (1)
7. 1-bromo-2,2-dimethyl-propane 3 (1) [7]
Important to remember:
- When naming haloalkanes, the halogen atoms do not get preferenceover alkyl groups – numbering should start from the side nearestto the first substituent, either the alkyl group or the halogen. Inhaloalkanes, where e.g. a Br and a Cℓ have the same number whennumbered from different sides of the chain, Br gets alphabeticalpreference.
- When writing IUPAC names, substituents appear as prefixes writtenalphabetically (bromo, chloro, ethyl, methyl), but the prefixes di- andtri- should not be used to determine the alphabetical order.
- In molecules where the functional group is ALWAYS on the firstcarbon (such as in the case of carboxylic acids, aldehydes there isNO number added to indicate the position of the fuctional group.(e.g. Ethanoic acid or pentanal.)
Activity 3
1. Which ONE of the following formulae represents an alkane?
- C2H2
- C3H4
- C3H6
- C3H8 (2)
2. An organic compound has the structural formula shown below: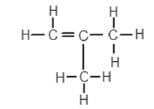
The correct systematic (IUPAC) name for the compound is …
- but-1-ene.
- but-2-ene.
- methylpropene.
- methylpropane.
3. Which ONE of the following compounds can exist as an isomer?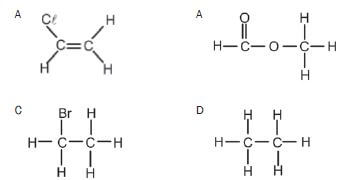 (2)
(2)
An example of an unsaturated hydrocarbon is:
- C2HCℓ3
- C3H6
- C2H6
- C2H5OH (2) [8]
Solutions
- D ✓✓ (2)
- C ✓✓ (2)
- B ✓✓ (2)
- B ✓✓ (2) [8]
Activity 4
Consider the following list of organic compounds: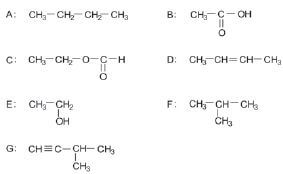
1. Using structural formulae, write an equation for the preparation of an ester. Choose the reactants from the above list. (5)
2. Write the letters representing TWO compounds in this list that are isomers. (2)
2.1 C
2.2 D (2)
3. Write down the letter that represents the compound that is formed when ethanol is oxided. (1)
4. Give one use of esters. (1) [11]
Solutions
1. 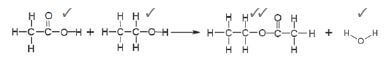
2. A ✓ & F ✓ (5)
2.1 ethylmethanoate ✓ (2)
2.2 but-2-ene ✓ (2)
3. B ✓ (1)
4. Manufacturing of perfumes/flavourants ✓ (1) [11]
1.2 Physical properties and structure
The structure of an organic compound — that includes the order in which atoms are connected in the compound, as well as the forces that is present between different molecules — has an influence on the physical properties of the compound. These physical properties include boiling points, melting points and the vapour pressure above a liquid organic compound. In the following section the influence of the structure of molecules on its physical properties will be discussed.
1.2.1 Intermolecular forces in organic compounds
Key for this section:![]()
Intermolecular forces that act between DIFFERENT molecules of a compound are called Van der Waals forces. In the organic chemistry that we study, there are primarily two kinds of forces, namely Hydrogen bonds and Dipole-dipole forces.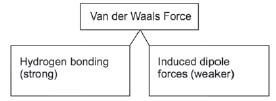
Induced dipole force: When two molecules come very close to each other, the electrons move and the distribution of electrons in the molecules temporarily shifts so that there is a slightly positive side and a slightly negative side on each molecule. The two molecules attract each other with a weak force.
Note:
The sketches that represent the forces between atoms relative to the size of the atoms are NOT drawn to scale. In reality, molecules are very close to each other and the intermolecular forces act over small distances.
Dipole-dipole forces: When a molecule has a slightly positive side and another molecule has a slightly negative side, the two molecules will attract each other with a weak force.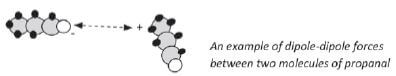
Hydrogen bonds: A stronger type of dipole-dipole force. The definition of hydrogen bonding was discussed thoroughly in Grade 11, but for the sake of the organic structures studied in Grade 12 it is helpful to observe that hydrogen bonds are mostly found between different molecules where at least one molecule has an –OH group (hydroxide group). Hydrogen bonds are present between the -O atom connected to one molecule (which is slightly negative) and the -H atom of a different molecule (that is slightly positive).
- Intermolecular bonds form between molecules so that they form liquids or solids.

e.g. Worked example 9
Identify the intermolecular forces found in propane.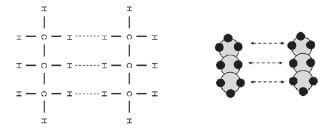
Solution
The intermolecular forces found between the molecules of propane are induced dipole forces.
The Van der Waals forces (induced dipole forces in this case) between the molecules are very weak, and are easily broken. This leads to the alkanes’ relatively low boiling points.
e.g. Worked example 10
Identify the intermolecular forces found in water.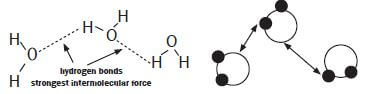
Solution
The intermolecular forces found between the molecules of water are hydrogen bonds.
The water molecules are strongly attracted to each other by the hydrogen bonds. Water has a relatively high boiling point due to the presence of the hydrogen bonds.
e.g. Worked example 11
Identify the intermolecular forces found between the molecules of ethanol.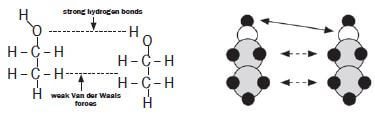
Solution
There are hydrogen bonds between the hydroxyl groups (–OH) of the different molecules. There are dipole-dipole forces between the hydrogen molecules connected to the different molecules.
Both hydrogen bonds and dipole-dipole forces exist between alcohol molecules. The hydrogen bonds exist between the polar OH– groups and the dipole-dipole forces exist between the non-polar hydrocarbon parts.
e.g. Worked example 12
Identify the intermolecular forces present in methanoic acid.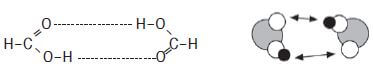
Solution
There are hydrogen bonds between the molecules of methanoic acid.
Intermolecular forces between carboxylic acids are stronger than those in alcohol because the molecules are bonded by two hydrogen bonds.
NB: DEFINITIONS
Vapour pressure: The vapour pressure of a liquid is the equilibrium pressure of a vapour above its liquid; that is, the pressure of the vapour resulting from evaporation of a liquid above a sample of the liquid in a closed container.
Boiling point: The temperature at which the vapour pressure of a substance equals atmospheric pressure. The stronger the intermolecular forces, the higher the boiling point. The boiling point of a substance is the temperature at which it changes from a liquid to a gas and is reached when the vapour pressure above the liquid equals the atmospheric pressure.
Melting point: The melting point of a substance is the temperature at which it changes from a solid to a liquid.
1.3 Physical properties of organic compounds
- Each chemical compound has physical properties such as its phase, melting point, boiling point, vapour pressure, viscosity, density and solubility. Note that the type of bonds affect all these properties.
- These properties are affected by the strength of the intermolecular forces between the molecules in the compounds.
When the intermolecular forces between the molecules of a substance arestrong:
- a lot of energy is required to overcome these intermolecular forces ofattraction;
- the molecules aren’t easily separated from one another.
NB: REMEMBER
- In general:
As the strength of the intermolecular forces INCREASES: - Less vapour is produced hence vapour pressure above the liquid DECREASES;
- Melting and boiling points INCREASE.
1.4 Factors that have an influence on the physical properties of organic compounds
In the Grade 12 exam you will be asked to explain why the physicalproperties (melting point, boiling point, vapour pressure) of differentorganic compounds, differ.In order to determine why the physical properties differ, you will need tofind answers to one or more of the following questions:
1. What is the influence of the type of intermolecular forces present between the molecules of the organic compound on its physical properties?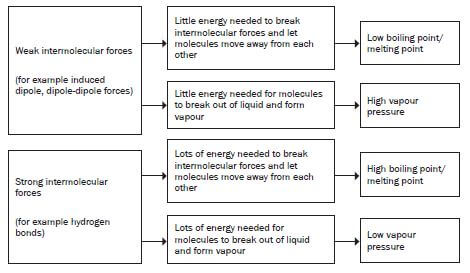
2. What is the influence of the type of functional group that is found in the molecule on its physical properties?
In organic compounds where hydroxyl groups (-OH) are present (for example alcohols, carboxylic acids) you will find hydrogen bonds and induced dipole forces.
In organic compound where hydroxyl groups are not present (for example alkanes, alkenes, alkynes, halo-alkanes, aldehydes, ketones and esters) you will find induced dipole forces or dipole-dipole forces.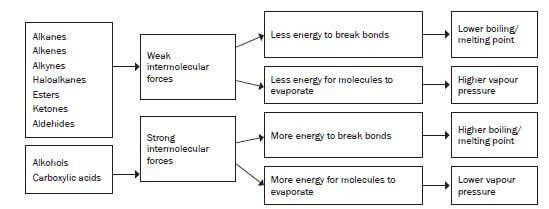
3. What is the influence of the chain length of the molecule on its physical properties?
As the length of the carbon chain in the organic compound increases, the number of sites where you will find the intermolecular forces will increase. Therefore, the longer the carbon chain, the stronger the intermolecular forces between the molecules.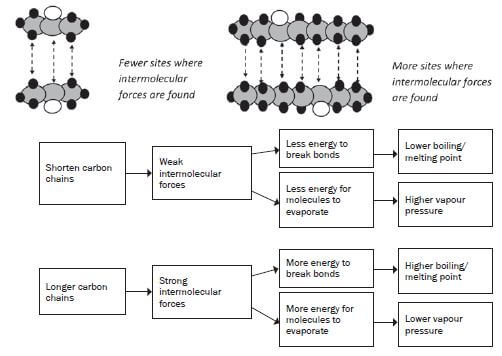
4. What is the influence of the molecular mass of an organic4. What is the influence of the molecular mass of an organiccompound on its physical properties?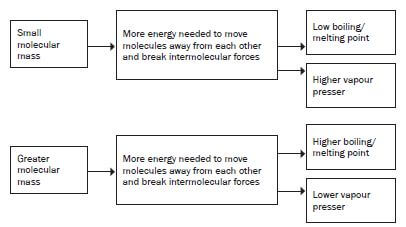
5. What will the influence on the physical properties of an organic compound be if the compound is made up of branched carbonchains?
As the number of branches (alkyl groups) increases, the shape of the molecule changes to a more compact, spherical shape with a smaller surface area, resulting in a smaller contact area between the molecules and weaker net Van der Waals forces.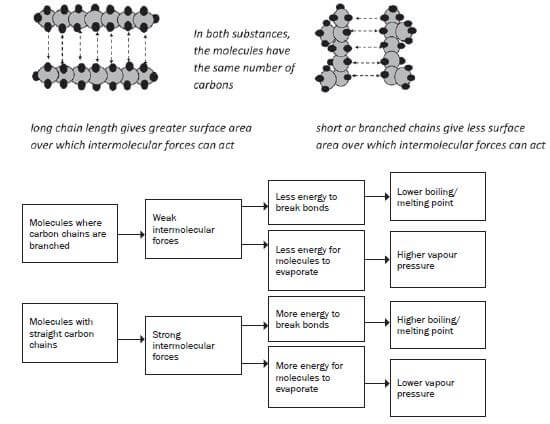
Activity 5
1.1 The boiling points of branched alkanes are lower than those of straight chain alkanes containing the same number of carbon atoms because branched alkane chains have
- Large molecular masses
- Longer chain lengths
- More electrons
- Smaller effective molecular surface areas (2)
1.2 Which ONE of these compounds has the highest vapour pressure at room temperature?
- Propane
- Ethane
- Ethanol
- Fluoroethane (2)
2. Will the boiling point of hexane be HIGHER THAN or LOWER THAN that of pentane? Refer to MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and ENERGY needed to explain the answer. (4)
3. How will the boiling point of an ISOMER of Butane compare to that of Butane? Write down HIGHER THAN, LOWER THAN or EQUAL TO. Refer to MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and the ENERGY needed to explain the answer. (4)
4. Consider the boiling points of compounds propan-1-ol (97˚C) and ethanoic acid (118˚C).
4.1 Give a reason for this difference in boiling points by referring to the intermolecular forces present in EACH of these compounds. (3)
4.2 Which ONE of the compounds propan-1-ol or ethanoic acid has a higher vapour pressure? Refer to their boiling points to give a reason for the answer. (3) [18]
Solutions
1.1 D ✓✓(2)
1.2 A ✓✓ (2)
- Higher ✓
Structure: Hexane has a longer chain length than pentane. OR Hexane contain more C- atoms than pentane. OR Hexane has a greater molecular size than pentane. OR Hexane has a larger surface area than pentane. ✓
Intermolecular forces: Between the different molecules of hexane you will find stronger or more intermolecular forces than between the different molecules of pentane. ✓
Energy: More energy needed to overcome or break intermolecular forces between hexane molecules than you need between pentane molecules. ✓ (4)
3. Lower than ✓
- Isomers of Butane: More branching / Smaller surface area (over which the intermolecular forces act.) ✓ Weaker/less intermolecular forces. ✓ Less energy needed to overcome intermolecular forces. ✓ (4)
4.
4.1 Ethanoic acid: Two sites for hydrogen bonding/forms dimers ✓ Propan-1-ol: One site for hydrogen bonding. ✓ Therefore it will require more energy to break the bonds between the ethanoic acid molecules than to break the bonds between
the propan-1-ol molecules, ✓ which explains why ethanoic acid has the lower boiling point. (3)
4.2 Propan-1-ol has the highest vapour pressure. ✓Because propan-1-ol has a lower boiling point it means less energy is required to break the bond between the particles of propan-1-ol ✓and therefore more molecules will be present in the vapour state and therefore vapour pressure will increase. ✓ (3) [18]
1.5 Solids, liquids and gases
Solids, liquids and gases are all phases of matter.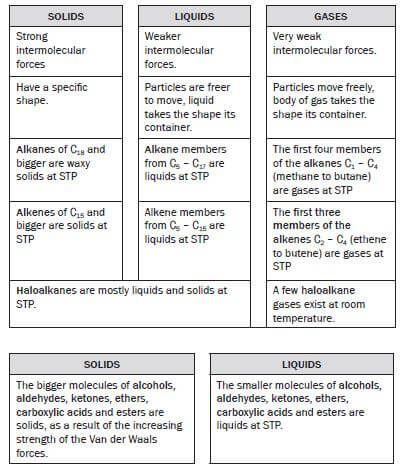
1.6 Chemical properties of organic compounds
Alkanes our most important fossil fuel and are good sources of heat and energy.
| 1.6.1 HYDROCARBONS | ||
| ALKANES CnH2n+2 | ALKENES CnH2n | ALKYNES CnH2n−2 |
| Are saturated hydrocarbons and therefore relatively unreactive. | Are unsaturated hydrocarbons and therefore reactive. | Are highly unsaturated hydrocarbons and therefore very reactive. |
|
|
|
| All are volatile, explosive and flammable and are used as fuels because their oxidation (with oxygen) is exothermic, and releases large amounts of energy. | ||
| Ethene is used in the manufacturing of plastic (polymer of ethane). | Ethyne used to be known as acetylene and is used on a large scale as fuel for the oxyacetylene blowtorch used to cut and weld metals. When ethyne burns in the presence of oxygen, a flame temperature of 2 800 °C can be reached. |
1.6.2 HALOALKANES | |
Are
| |
Are
The more halogen atoms on a haloalkane
| |
Tetrachloromethane CCℓ4 is
| Other haloalkanes are
|
DID YOU KNOW? By Jynto and Ben Mills [Public domain], via Wikimedia Commons | |
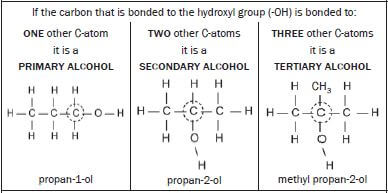
1.6.3 ALCOHOLS |
Are
|
Are
|
 |
|
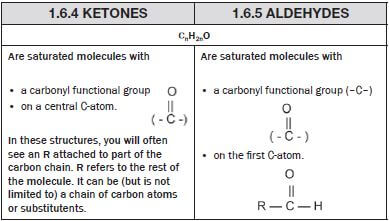 | |
Are
| Are
|
Intermolecular forces:
| Intermolecular forces:
|
Solubility in H2O:
| Solubility in H2O:
|
Propanone is also known as acetone and is used as nail polish remover. | |
1.6.6 CARBOXYLIC ACIDS | 1.6.7 ESTERS |
| CnH2nO2 | |
Are saturated molecules with
| Are saturated molecules with
|
Are
| Are
|
Intermolecular forces:
| Intermolecular forces:
|
Boiling points:
| Boiling points:
|
Usage and Occurrence:
| Used in the manufacture of:
|
NB: VOCABULARY
- rancid: old, stale and with an unpleasant smell
- preservative: prevents food from going off or decaying
- readily: easily
1.7 Reactions of organic compounds
1.7.1 Types of chemical reactions
Organic compounds take part in different types of reactions which can be grouped into:
- Oxidation
- Substitution
- Addition and
- Elimination
1.7.1.1 Oxidation reactions:
| Reaction with oxygen (O2) Exothermic (release energy) Excess O2 alkane + O2(g) → CO2(g) + H2O(g) + energy |
:1.7.1.2 Substitution reactions
- Take place when saturated compounds react and an atom bonded tothe carbon chain is substituted by another atom or another group ofatoms.
- Produce products which are also saturated
- Are slow.
- Are not spontaneous – additional energy (e.g. sunlight hf or heat Δ)is needed for the reactions to take place.
| SUBSTITUTION REACTIONS | Reaction conditions | Reaction equations |
| Halogenation | Endothermic: Sunlight or heat hf or ∆ | hf or ∆ alkane + halogen → haloalkane + HX |
| Hydrolysis | Endothermic VERY SLOW | haloalkane + H2O → alcohol + HX |
| Slow | haloalkane + NaOH (diluted) → alcohol + NaX |
NB: REMEMBER
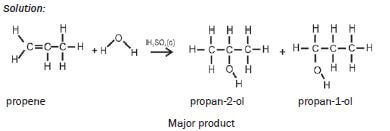
Markovnikov’s rule:
- Two products are formed during addition of water or of HX to an alkene.
- The major product is formed when the H-atom from the added molecule bonds to the C-atom which is already bonded to the most other H-atoms.
- The secondary product is formed when the H-atom from the added molecule bonds to the C-atom which is bonded to the least other H-atoms.
1.7.1.3 Addition reactions:
- Take place when atoms attach to the double or triple bond of anunsaturated compound (alkenes or alkynes), breaking the double ortriple bond during the reaction.
- Form products which are more saturated than the reactants.
- Are faster than substitution reactions.
- Are usually spontaneous
| ADDITION REACTIONS | REACTION CONDITIONS | REACTION EQUATIONS |
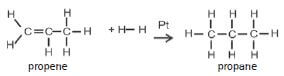
| Hydrogenation | Catalyst: Pt, Pd or Ni | Pt alkene + H2(g) → alkane |
| Halogenation | No catalyst | alkene + X2(g) → haloalkane |
| Hydrohalogenation | No catalyst | alkene + HX(g) → haloalkane |
| Hydration | Concentrated H2SO4 | H2SO4 (conc) alkene + H2O(g) → alcohol |
1.7.1.4 Elimination reactions:
- Occur when two atoms or groups of atoms are removed fromadjacent carbon atoms in a saturated compound (like an alkane, ahaloalkane or an alcohol) to form two compounds;
- are always endothermic i.e. the reactants must be heated.
| ELIMINATION REACTIONS | REACTION CONDITIONS | REACTION EQUATIONS |
| cracking | Catalyst (Pt), Heat | PtΔ alkane → alkene + alkane (long chains form shorter structures) |
| dehydrogenation | Catalyst (Pt), Heat | PtΔ alkane → alkene + H2 |
| dehydrohalogenation | Concentratedstrong base(NaOH), Heat | NAOH(e)Δ haloalkane → alkene + HX |
| dehydration | Concentrated H2SO4 , Heat | H2SO4(e)Δ alcohol → alkene + H2O |
1.8 Reactions of different homologous series
1.8.1 Reactions of the ALKANES
As alkanes are saturated hydrocarbons, they are relatively unreactive.
Alkanes burn in excess oxygen to produce carbon dioxide and in limited oxygen, to produce carbon monoxide. In both cases water vapour is produced and both reactions are exothermic (∆H < 0). Alkanes are therefore sources of energy and can be used as fuel.
STEPS to balance reaction equations for the oxidation of alkanes, alkenes andalkynes
- Write the standard reaction equation, with the correct formula for the alkane.
alkane + (excess) O2(g) → CO2(g) + H2O(g) + energy - Put a 2 in front of the alkane, alkene or alkyne.
- Balance the C-atoms on the right hand side.
- Balance the H-atoms on the right hand side.
- Balance the O-atoms on the left hand side.
- Check whether the balancing ratio is in the simplest form.
Zaitsev’s* rule:
If more than one elimination product is possible
- the major product is formed when the H-atom is removed from the C-atom with the least H-atoms bonded to it and
- the secondary product is formed when the H-atom is removed from the C-atom with the most H-atoms bonded to it.
* If you look this rule up online, you might find it is spelled ‘Saytseff’ as well.
e.g. Worked example 13
Write the balanced reaction for the reaction of ethane burning in excess oxygen, using molecular formulae.
Solution
C2H6(g) + O2(g) → CO2(g) + H2O(g) + energy (unbalanced)
2C2H6(g) + 7O2(g) → 4CO2(g) + 6H2O(g) + energy (balanced)
| SUBSTITUTION | HALOGENATION (+X2hf,Δ) |
|
Example: 1. Write a balanced reaction for the reaction of methane with chlorine gas, using molecular formulae. Name the products. |
| SUBSTITUTION | CRACKING (Δ, Pt) |
|
Examples:
|
1.8.2 Reactions of the ALKENES
| ADDITION | HYDROGENATION (+H2,Pt) | PtΔ
|
Examples: 1. Write an equation for the hydrogenation of etheneusing condensed structural formulae. Name theproduct. |
NB: Remember
- The reaction of a haloalkane with water to form an alcohol is called hydrolysis. Any splitting of a molecule by water is also called hydrolysis.
- However other reactions between organic compounds and water are called hydration reactions as the hydrogen from the water becomes part of the product.
NB: Remember
- elimination
Saturated compound → unsaturated compound
alkane / haloalkane / alcohol alkene - addition
Unsaturated compound → saturated compound
alkene alkane / haloalkane / alcohol
| ADDITION | HALOGENATION (+ X2) | alkene + X2 → haloalkane (X = Cℓ, Br, F, I)
|
| Examples: 1. Write an equation for the addition of bromine to ethene, using condensed structural formulae. Name the product. Solution: CH2=CH2 + Br2 → CH2Br–CH2Br ethene 1,2-dibromoethane 2. Write an equation for the addition of chlorine to propene, using structuralformulae. Name the product. Solution: 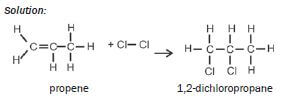 |
| ADDITION | HYDROALOGENATION (+ HX) | alkene + HX → haloalkane (X = F, Cℓ, Br, I)
| ||
| Examples: 1. Write an equation for the reaction between ethene and hydrogen fluoride, usingcondensed structural formulae. Name the product. Solution: CH2=CH2 + HCℓ → CH3CH2Cℓ ethene chloroethane 2. Write an equation for the reaction of propene with hydrogen chloride(hydrochloric acid), using structural formulae. Name the two products andidentify the major product. Solution: 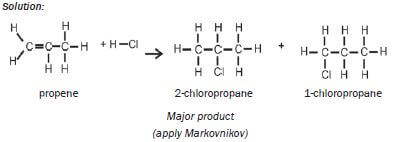 |
| ADDITION | HYDRATION (+ H2O, H2SO4 concentartion) | H2SO4 (conc)
HINT! Examples: |
1.8.3 Reactions of the HALOALKANES
| SUBSTITUTION | WITH DILUTE STRONG BASE (NOH OE KOH) TO PRODUCE AN ALCOHOL | haloalkane + NaOH (dilute) → alcohol + NaX
|
| Examples: 1. Write an equation for the reaction of bromo methane with a dilute sodiumhydroxide solution, using condensed structural formulae. Name the products. Solution: CH3Br + NaOH → CH3OH + NaBr bromo methane methanol sodium bromide 2. Write an equation for the reaction of 2-bromopropane with a dilute potassiumhydroxide solution, using structural formulae. Name the products. Solution: 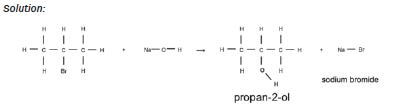 |
NB: Remember
The reaction of a haloalkane with water to form an alcohol is called hydrolysis. Generally, splitting any molecule with water is also called hydrolysis.
However other reactions between organic compounds and water are called hydration reactions, particularly when OH is added to the compound.
NB: VOCABULARY
Heated under reflux: A solution is heated and the vapour is cooled down so that it condenses and return to the reaction vessel.
| SUBSTITUTION | HYDROLYSIS WITH WATER (H2O) TO PRODUCE ALCOHOLS | Haloalkane + H2O → alcohol + HX
|
Example:
Solution:
Solution: |
| ELIMINATION | DEHYDROHALOGENATION (NaOH, (c), Δ) | NaOH(c)Δ
Apply Zaitsev’s rule as discussed earlier, in order to determine the majorproduct! |
| Example:Write an equation for the reaction of 2-chlorobutane with concentrated sodiumhydroxide when heated under reflux, using structural formulae. Show the majororganic product only and name it and the by-product. Solution: 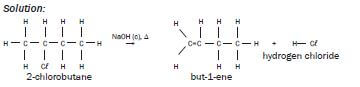 |
1.8.4 Reactions of ALCOHOLS
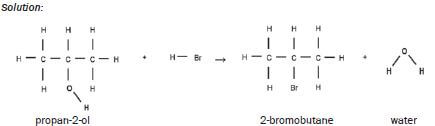
| SUBSTITUTION | HALOGENATION (+NaX) | alcohol + NaX → haloalkane + H2O
|
Example: Solution: |
| ELIMINATION | DEHYDRATION (H2SO4 conc) | H2SO4(c)∆
hint Apply Zaitsev’s rule as discussed earlier, in order to determine the major product! |
Example: |
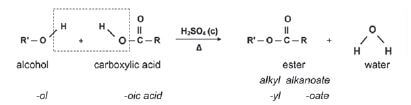
1.8.5 Reactions of ESTERS
| ESTERIFICATION (alcohol + carboxylic acid, H2SO4 concentrated) |
|
Examples: |
hint Remember
- Reaction conditions in Addition, Elimination and Substitution
- The role of Sulphuric acid in Esterification, Addition & Elimination
1.9 Creating one hydrocarbon from another
The diagram below illustrates how to use Elimination, Substitution and Addition reactions to create one hydrocarbon from another.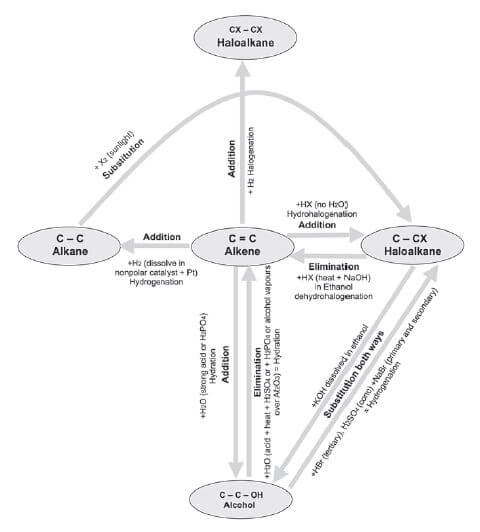
Activity 6
1. Unsaturated vegetable oils are hardened to make margarine. The reaction that takes place during the hardening process can be described as:
- Addition
- Hydrogenation
- Substitution
- I and II
- II and III
- Only I
- Only II (2)
2. Which ONE of the following compounds has the highest melting point?
- Butane
- Butene
- 1-bromobutane
- 1-methylpropane (2)
3. Which ONE of the following pairs of compounds correctly represents the products formed during the COMPLETE combustion of Octane?
- CO and H2O
- CO and H2
- CO2 and H2
- CO2 and H2O (2)
4. Most organic compounds can undergo substitution or addition or elimination reactions to produce a variety of organic compounds. Some incomplete reactions are shown below: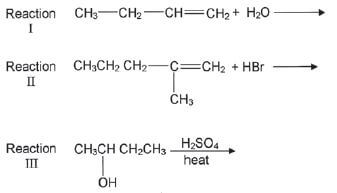
4.1 Name the type of reaction represented by Reaction III. (1)
4.2 Both Reactions I and II are examples of addition reactions. Name the type of addition reaction that is represented by each reaction. (2)
4.3 Write down the IUPAC name of the major product formed in Reaction I. (1)
4.4 Reaction I only takes place in the presence of a catalyst. Write down the formula of the catalyst used in Reaction I. (1)
4.5 Write down the structural formula and the IUPAC name of the major product in Reaction II. (3)
4.6 To which homologous series does the organic product formed in Reaction III belong? (1) [15]
Solutions
1. A ✓✓ (2)
2. C ✓✓ (2)
3. D ✓✓ (2)
4.1 Elimination ✓ (1)
4.2 I- hydration ✓ II- hydrohalogention 3 (2)
4.3 Butan-2-ol ✓ (1)
4.4 H2SO4 ✓ (1)
4.5 2-bromo-2-methylpentane ✓ (3)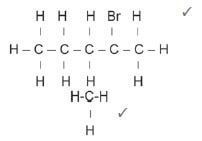
4.6 Alkene ✓ (1) [15]
1.10 Plastics and polymers
1.10.1 Polymers
NB: DEFINITION
- Polymers: are a type of macromolecule which form when monomers bond to each other chemically to produce large molecules that are built up of repeating units in chains that vary in length depending on the number of monomers that bond.
- Many polymers occur in nature (wood, carbohydrates, proteins etc.) while a large number are produced synthetically (most are plastics) as they are very useful.
1.10.2 Synthetic polymers: Plastics
- All contain carbon in their basic structure.
- Are light because they have a less dense structure than other solids.
- Longer polymers have stronger intermolecular forces between them.
- Cross linking between molecules increases their strength.
- Do not conduct heat or electricity.
- Are versatile and can be used as foam, can be moulded or extruded into fibres.
Synthetic polymers (plastics) can be divided into two main types, namely thermoplastic and thermoset polymers.
THERMOPLASTIC POLYMERS | THERMOSET POLYMERS |
| Can be softened by heating and hardened by cooling repeatedly therefore can be remoulded into different shapes and so can be recycled. | Are melted when they are made and set into a hard insoluble mass but can’t be softened again and therefore can’t be remoulded into different shapes, so can’t be recycled. |
| Consists of long chains that slide past each other when melted. | Cross-llinks in the molecule prevent molecules from sliding past each other. |
 |  |
 |  |
1.10.3 Properties of plastics, nylons and polyesters
| Plastics | Nylons | Polyesters |
| Are formed by addition polymerisation | Are formed by condensation polymerisation between a dicarboxylic acid and a diamine. | Are formed by condensation polymerisation between a dicarboxylic acid and a diol. |
|
|
|
Examples:
| Examples:
| Examples:
|
Properties of polymers are determined by the intermolecular forces between the chains.
Polymers
- with carbonyl groups can form strong hydrogen bonds and are strong with high melting points;
- like polyesters have dipole-dipole bonds (that are weaker than hydrogen bonds) between the chains, so have lower melting points and are more flexible (bendable);
- with only Van der Waals forces between the chains have low melting points and are flexible.
10.4 PolymerisationPolymerisation
- is a chemical process in which monomers combine to form polymers;
- takes place in three steps:
- initiation (the step that starts the reaction)
- propagation (the steps that allow the reaction to continue) and
- termination (the step that ends the reaction).
- is either addition polymerisation or condensation polymerisation.
ADDITION POLYMERISATION | CONDENSATION POLYMERISATION |
|
|
|
|
Example:
| Example:
|
|
|
1.10.5 Addition polymerisation of ethene to1.10.5 Addition polymerisation of ethene toproduce polyethene
Example 1:
(Note: This is the only addition polymerisation example you need to learn.)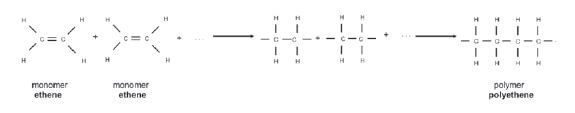
- The length of the polyethene macromolecule depends on the number of ethene monomers that bond.
- The structural formula of the polymer polyethene can therefore be abbreviated as follows:
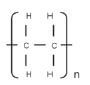 where n is the number of monomers that joined.
where n is the number of monomers that joined.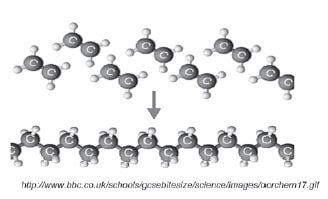
| There are two types of polyethene | ||
| Low density polyethene LDPE | High density polyethene HDPE | |
| Relative strength | WEAK | STRONG |
| Number of branches on the polymer
| MANY | FEW |
| Maximum usable temperature (°C) | 85 | 120 |
| Uses: |  | |
| ||
1.10.6 Condensation polymerisation to produce a polyester
- The alcohol has a hydroxyl group on each end of its carbon chain, so it is a -diol.
- A H-atom is removed from the hydroxyl group on the alcohol and a hydroxyl group is lost from the dicarboxylic acid.
- The −H and the −OH bond to form a water molecule, H2O.
- A new bond forms between the O-atom on the alcohol and the C-atom on the carboxylic acid. This is an ester linkage.
- The carboxyl group on the other end of the dicarboxylic acid can now react further with another -diol, while the hydroxyl on the other end of the -diol can now react with another dicarboxylic acid.
- This results in a long chain-like compound – a polymer.
- This polymer is known as polyester.
Example: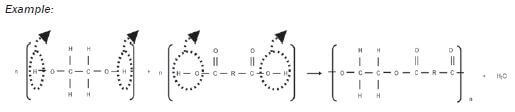
- There are many different polyester fibres with a wide range of uses. Kevlar and Mylar are two examples.
KEVLAR | MYLAR | |
Properties |
|
|
Uses |
|
|
 | ||
1.11 Plastics and pollution
1.11.1 Disadvantages of polymer usage
Although synthetic plastics are light, easy to shape and relatively cheap, there is widespread opposition to the use of these materials.
- Most are not bio-degradable.
- When burned to dispose of them, poisonous gases may be released.
- Landfill sites use up land and fill quickly.
- Litter causes ecological problems.
11.2 Recycling
- Biodegradable plastics would solve most problems, but areexpensive.
- Responsible recycling is essential.

Problems with Recyclability:
- Not possible in very rural areas.
- High costs.
- Not all plastics can be recycled.
- Communities are not all aware of the problems.
| High density polythene (HDPE) | Low density polythene (LDPE) |
 | |
Recycled to produce
| Recycled to produce
|
Activity 7
1. Give the definition of a macromolecule (1)
2. Explain the difference between a monomer and a polymer. (2)
3. What is the chemical reaction in which a monomer molecules join to form a polymer called? (1)
4. The polymer below is the product of a polymerisation reaction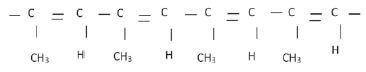
4.1 What is the IUPAC name of the monomer used to form this polymer? (1)
4.2 Give the structural formula of the monomer used to form this polymer. (2)
4.3 Is this an example of an addition or condensation polymerisation? Give a reason for your answer. (2)
5. A carboxylic acid monomer and an amine monomer has joined in an amide linkage as shown below.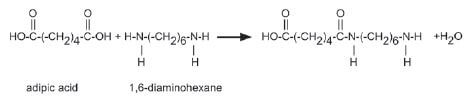
Name the type of polymerisation that occurs in this equation. Give a reason for your answer. (2)
6. Write an equation for polymerisation of ethane to form polyethene. (2)
7. Is (6) an example of addition or condensation polymerisation? Give a reason for your answer. (2)
8. Name at least three industrial uses of polythene. (3) [18]
Solutions
1. A molecule that consists of a large number of atoms. ✓ (1)
2. A polymer is a large molecule that is made up of smaller units, covalently bonded to each other in a repeating pattern. ✓Each of these smaller units are called a momomere. ✓ (2)
3. Polymerisation ✓ (1)
4. 4.1 prop-(1)-yne (1)
4.2 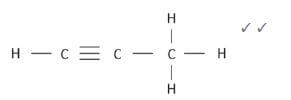 (2)
(2)
4.3. Addition polymerisation 3 monomers combined through an addition reaction. ✓ (2)
5. Condensation polymerisation. Two monomers with different functional groups are joined ✓ and when they join it leads to the “loss” of a small molecule, in this case, water. ✓ (2)
6. 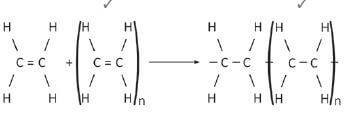 (2)
(2)
7. Addition polymerisation ✓ monomers combinded through an addition reaction. ✓ (2)
8. Any of the following: sandwich bags, ✓cling wrap, ✓ car covers, ✓ squeeze bottles, liners for tanks and ponds, moisture barriers in construction, freezer bags, water pipes, wire and cable insulation, extrusion coating. (3) [18]