ACIDS AND BASES GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
Share via Whatsapp Join our WhatsApp Group Join our Telegram Group- Summary
- Properties of acids and bases
- Common acids
- Common bases
- Mono- and polyprotic acids
- Conjugate acid-base pairs
- Ampholyte (amphiprotic) substance
- Salt hydrolysis
- Acid-base indicators
- Acid-base titrations (volumetric analysis)
- Preparing a standard solution
- Dilution of solutions
- Acid-base titration calculations
- The Equilibrium Constant (Kc) (The Law of Mass Action)
- Ka and Kb values
- The relationship between Ka and Kb for a substance
- Auto-ionisation of water
- Equilibrium constant for water (Kw)
- The pH scale
- pH Calculations for strong acids and bases
- Summary: Strong and weak acids and bases
- Summary: Concentrated and dilute acids and bases
Acids and bases
4.1 Summary
| Acid | (Arrhenius theory): is a substance that produces hydrogen ions (H+) / hydronium ions (H3O+) when it dissolves in water (Brønsted-Lowry theory): is a proton (H+ ion) donor. |
| Strong acid | Ionises completely in water to form a high concentration of H3O+ ions. Examples of strong acids are hydrochloric acid (HCℓ), sulphuric acid (H2SO4) and nitric acid (HNO3). |
| Weak acid | Ionises incompletely in water to form a low concentration of H3O+ ions. Examples of weak acids are ethanoic acid (CH3COOH) and oxalic acid (COOH)2. |
| Concentrated acid | Concentrated acids contain a large amount (number of moles) of acid in proportion to the volume of water. |
| Diluted acid | Dilute acids contain a small amount (number of moles) of acid in proportion to the volume of water. |
| Base | (Arrhenius theory): is a substance that produces hydroxide ions (OH–) when it dissolves in water. (Brønsted-Lowry theory): is a proton (H+ ion) acceptor. |
| Strong base | Dissociates (breaks up) completely in water to form a high concentration of OH– ions. Examples of strong bases are sodium hydroxide (NaOH) and potassium hydroxide (KOH). |
| Weak base | Dissociates/ionises incompletely in water to form a low concentration of OH– ions. Examples of weak bases are ammonia (NH3), calcium carbonate (CaCO3), potassium carbonate (K2CO3) , and sodium hydrogen carbonate (NaHCO3). |
| Concentrated base | Concentrated bases contain a large amount (number of moles) of base in proportion to the volume of water. |
| Diluted base | Dilute bases contain a small amount (number of moles) of base in proportion to the volume of water. |
| Equivalence point | is the point at which the acid / base has completely reacted with the base/acid. |
| End point | is the point where the indicator changes colour. |
| Ionisation | a process that takes place when a covalent compound reacts with water to form new ions OR Breaking up of a molecule into charged components (ions). In acid-base reactions this usually means dissolving in water. |
| Dissociation | a process that take place when an ionic compound dissolves in water allowing the ions in the compound to separate. The same as ionisation. |
| Hydrolysis | ionisation of a salt in water, or, more generally, splitting a molecule by reacting it with water (e.g. in organic chemistry). |
| Ampholyte (Amphiprotic) | is a substance that can act either as an acid or a base. example: Water (H2O) |
Activity 1
1. An Arrhenius acid is a substance that
- Accept a proton
- Donates a proton
- Produces H+ in an aqueous solution
- Produces OH– in an aqueous solution (2)
2. Which of the following is an example of the strong base
- CaCO3
- KOH
- K2CO
- NaHCO3 (2)
An aqueous solution that contains more hydronium ions than hydroxyl ions is a(an)
- Acidic solution
- Neutral solution
- Basic solution
- Standardised solution (2)
A solution that has a large amount of dissolved substances in proportion to the volume of water
- Strong solution
- Weak solution
- Concentrated solution
- Diluted solution (2) [8]
Solutions
- D✓✓
- B ✓✓
- A✓✓
- C✓✓ [8]
4.2 Properties of acids and bases
| ACIDS | BASES | |
| PROPERTIES |
|
|
| ACID-BASE THEORIES | ARRHENIUS | |
|
| |
| BRØNSTED-LOWRY | ||
|
| |
| REACTIONS WITH WATER |
|
|
NB: NOTE:
You must know the Arrhenius and the Brønsted-Lowry definitions for acids and bases, BUT we will ALWAYS use the Brønsted-Lowry theory for all further discussions of acids and bases.
Activity 2
1. Which of the following is the property of an acid
- Decreases H3O+ ion concentration in solution
- Decreases OH– ion concentration in solution
- Increases OH– ion concentration in solution
- Increases the pH of a solution (2) [2]
Solution
1. B✓✓ [2]
4.3 Common acids
| ACID | FORMULA | STRONG / WEAK | EXAMPLES & USES | ||
| Hydrochloric acid | HCℓ | Strong | IONISES ALMOST COMPLETLY IN WATER |
|
| Sulphuric acid | H2SO4 | Strong | Used
Used
| ||
| Nitric acid | HNO3 | Strong | Used
| ||
| Oxalic acid | (COOH)2 | Weak | IONISES PARTIALLY IN WATER | Used
| |
| Phosphoric acid | H3PO4 | Weak | Used
| ||
| Ethanoic acid | CH3COOH | Weak | Vinegar Used
| ||
| Carbonic acid | H2CO3 | Weak | Used
|
Hint:
- Strong acid: HCℓ; H2SO4; HNO3
- Weak acid: CH3COOH; (COOH)2
- Strong base: NaOH; KOH; Mg(OH)2
- Weak base: NH3; NaHCO3 ; CaCO3
4.4 Common bases
| BASE | FORMULA | STRONG/WEAK | EXAMPLES & USES | ||
| Sodium hydroxide (Caustic soda) | NaOH | Strong | DISSOCIATES COMPLETLY IN WATER | Used
|
| Potassium hydroxide (Caustic potash) | KOH | Strong | Used
| ||
| Magnesium hydroxide | Mg(OH)2 | Strong | Used
| ||
| Calcium hydroxide (Slaked lime) | Ca(OH)2 | Strong | Used
| ||
| Sodium carbonate (Washing soda) | Na2CO3 | Weak | DISSOCIATES / IONISES PARTIALLY IN WATER | Used
| |
| Calcium carbonate (Limestone) | CaCO3 | Weak | Found in marble and sea shells. Used
| ||
| Ammonia | NH3 | Weak | Used
| ||
| Sodium bicarbonate (Baking soda) | NaHCO3 | Weak | Used
|
4.5 Mono- and polyprotic acids
Acids can be classified according to the number of protons (H+) that they can donate.
NB: Monoprotic acids have only one proton (H+) to donate. Polyprotic acids can donate two or three protons. The protons are donated in steps as shown in the examples in the table below.
Monoprotic acids | Polyprotic acids Can donate more than one proton (H+) | |
Diprotic acids | Triprotic acids | |
| HCℓ, HNO3, CH3COOH | H2SO4, H2CO3 | H3PO4 |
E.g. | E.g. | E.g. H3PO4 → H+ + H2PO4− H2PO4− → H+ + HPO42− HPO42− → H+ + PO43− |
4.6 Conjugate acid-base pairs
4.6.1 Acid-base reactions
hint “Conjugate” is from Latin, it means literally “yoked together” or to be a couple.
Acid-base reactions take place simultaneously. The acid donates a proton to the base, while the base accepts the proton from the acid.
NB: DEFINITION
An acid-base (protolytic) reaction is a reaction in which a proton (H+) is transferred
4.6.2 Conjugate acids and bases
CONJUGATE ACIDS | CONJUGATE BASES |
When an acid donates a proton (H+), a conjugate base is produced. | When a base receives a proton (H+), a conjugate acid is produced. |
| acid ⇌ H+ + conjugate base | base + H+ ⇌ conjugate acid |
| Examples: HCℓ ⇌ H+ + Cℓ− acid conjugate base H2SO4 ⇌ H+ + HSO4− acid conjugate base | Examples: OH− + H+ ⇌ H2O base conjugate acid HSO4− + H+ ⇌ H2SO4 base conjugate acid |
Activity 3
1. In the reaction: H2SO4(aq) + H2O(ℓ) ⇌ HSO4–(aq) + H3O+(aq), the Brønsted-Lowry bases are:
- H2O and H3O+
- H2SO4 and H3O+
- HSO4– and H3O+
- H2O and HSO4– (2) [2]
Solution
1. D ✓✓ [2]
Activity 4
Find the conjugate bases and conjugate acids.
| Acid | Conjugate base | Base | Conjugate acid |
HCℓ | Cℓ− | ||
HNO3 | NO3− | ||
H2SO4 | HSO4− | ||
HSO4− | SO42− | ||
H3PO4 | H2PO4− | ||
H2PO4− | HPO42− | ||
HPO42− | PO43− | ||
H2CO3 | HCO3− | ||
HCO3− | CO32− | ||
CH3COOH | SO42− | ||
(COOH)2 | HSO4− | ||
H2O | OH− | ||
NH4+ | NH3 | ||
H3O+ | H2O |
[28]
Note that some of these are marked in BOLD. That is because they are “amphiprotic”. We will see what this means later on.
Solution
1. Check your answers
Acid | Conjugate base |
HCℓ | Cℓ− |
HNO3 | NO3− |
H2SO4 | HSO4− |
HSO4− | SO42− |
H3PO4 | H2PO4− |
H2PO4− | HPO42– |
HPO42− | PO43− |
H2CO3 | HCO3− |
HCO3− | CO32− |
CH3COOH | CH3COO− |
(COOH)2 | C2O4H− |
H2O | OH− |
NH4+ | NH3 |
H3O+ | H2O |
Base | Conjugate acid |
Cℓ − | HCℓ |
NO3− | HNO3 |
HSO4− | H2SO4 |
SO42− | HSO4− |
H2PO4− | H3PO4 |
HPO42− | H2PO4− |
PO43− | HPO42− |
HCO3− | H2CO3 |
CO32− | HCO3− |
SO42− | HSO− |
HSO4− | H2SO4 |
OH− | H2O |
NH3 | NH4+ |
H2O | H3O+ |
[28]
Steps to follow when identifying conjugates
- Find the acid on the left hand side of the arrow. Label it acid1.
- Find the conjugate base of this acid on the right hand side of the arrow. Label it base1.
- Draw a bracket to show that these two form an acid-base conjugate pair.
- Find the base on the left hand side of the arrow. Label it base2.
- Find the conjugate acid of this base on the right hand side of the arrow. Label it acid2.
- Draw a bracket to show that these two form an acid-base conjugate pair.
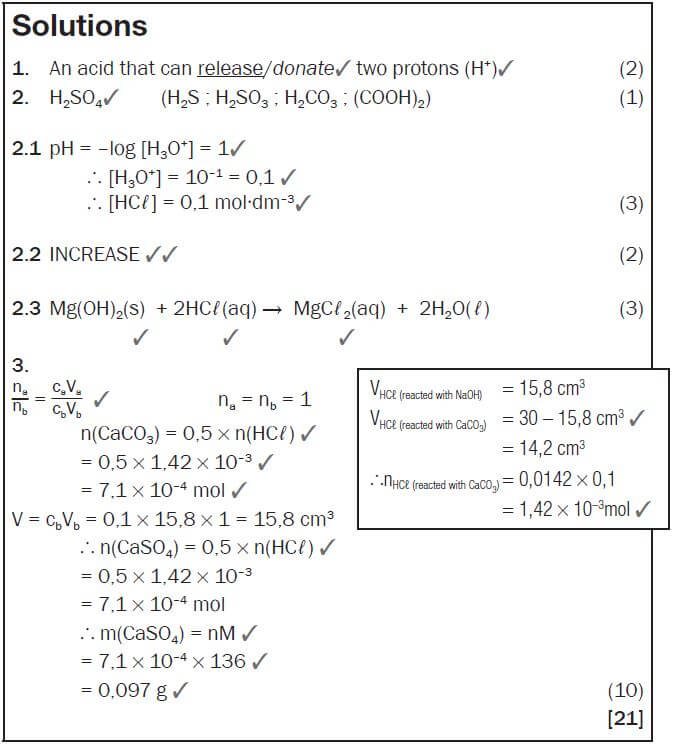
e.g. Worked example 1
For each of the following reactions, indicate the acid-base conjugate pairs.
- HCℓ(g) + NH3(g) ⇌ Cℓ−(aq) + NH4+(aq)
- H2SO4(g) + H2O(ℓ) ⇌ HSO4−(aq) + H3O+(aq)
- H3PO4(g) + OH−(aq) ⇌ H2PO4−(aq) + H2O(ℓ)
Solutions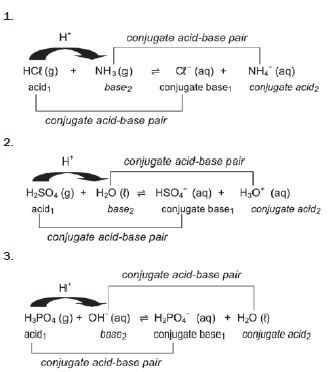
4.7 Ampholyte (amphiprotic) substance
An ampholyte:
- acts as a base in the presence of an acid and
- acts as an acid in the presence of a base.
NB: Ampholyte is a substance that can act as either a base or acid.
Example:
Water (H2O) is an ampholyte:
Water as an acid | Water as a base |
H2O(ℓ) + NH3(g) ⇌ OH−(aq) + NH4+(aq) | HCℓ(g) + H2O (g) ⇌ Cℓ−(aq) + H3O+(aq) |
Example:
Show that hydrogen sulphate ion (HSO4−) is an ampholyte.
The hydrogen sulphate ion (HSO4−) donates a proton when it acts as an acid | The hydrogen sulphate ion (HSO4−) accepts a proton when it acts as a base |
HSO4−(aq) ⇌ H+(aq) + SO42−(aq) | HSO4−(aq) + H+(aq) ⇌ H2SO4(aq) |
Activity 5
In the acid-base equilibrium formed by adding HSO4– and OH– the acids are:
- HSO4– and H2SO4
- HSO4– and H2O
- SO42– and H2SO–
- SO42– and H2O (2)
Which of the following is amphiprotic in water?
- SO2
- SO32–
- HSO3–
- H2SO3 (2) [4]
Solutions
1. B✓✓ 2. C✓✓ [4]
4.8 Salt hydrolysis
A salt is formed in the reaction between an acid and a base. When an ionic salt dissolves in water, the ions in the salt dissociate. These ions react with the water and new ions form.
NB: Hydrolysis occurs when a salt (a compound made of a metal + non-metal portion) reacts with water. Hydrolysis, more generally, is the splitting of any compound by reacting it with water. In this chapter, we only deal with the hydrolysis of salts. (Salt is not just what you have on your table; that is just one type of salt, specifically NaCℓ. Any metal combined with any non-metal is a salt).
e.g. Worked example 2
To determine whether the solutions of each of the following salts are:
- acidic or basic, and the pH of each solution is above or below 7?
- NH4NO3(aq) (2)
- K2SO4(aq) (2)
Solutions
In summary:
Remember that acids and bases react with each other to form new compounds:
We can determine whether the salt solution is basic or acidic by comparing the strengths of the reacting acids and bases.
- A salt formed between a strong acid and a weak base is an acidic salt, for example NH4Cℓ.
- When a salt reacts with water to form hydronium ions (H3O+), the solution is acidic (pH < 7).
- A salt formed between a weak acid and a strong base is a basic salt, for example NaCH3COO.
- When a salt reacts with water to form hydroxyl ions (OH−), the solution is basic (pH > 7).
- Neutral salt is formed when a strong acid and a strong base are neutralized in the reaction.
To determine the approximate pH of salts in salt hydrolysis
H2O forms H3O+ | H2O forms OH− |
|
|
Hydrolysis reaction: | Hydrolysis reaction: |
REMEMBER:
- When water reacts with a salt to form hydronium ions (H3O+), the solution is acidic (pH < 7).
- When a salt reacts with water to form hydroxyl ions (OH–), the solution is basic (pH > 7).
- The ions that don’t react are called spectator ions.
- Positive spectator ions: cations from Groups I and II e.g. K+ and Mg2+
- Negative spectator ions e.g. SO42–; Cℓ–; NO3–.
| Salt of | Nature of solution
| pH in an aqueous solution | Example | |
| Acid | Base | |||
| Strong | Strong | Neutral | pH = 7 | NaCℓ(aq) (HCℓ + NaOH) |
| Weak | Weak | Neutral | pH = 7 | CH3COONH4(aq) (CH3COOH + NH3) |
| Strong | Weak | Acidic | pH < 7 | NH4Cℓ(aq) (HCℓ + NH3) |
| Weak | Strong | Basic | pH > 7 | CH3COONa(aq) (CH3COOH + NaOH) |
Step by step
Step 1: Write down the formula of the acid and of the base that reacted to form the salt.
Step 2: Write down whether the acid is strong or weak.
Step 3: Write down whether the base is strong or weak.
Step 4: Is the salt solution acidic or basic?
e.g. Worked example 3
Determine whether each of the following salt solutions are acidic, basic or neutral.
a) Na2SO4(aq) b) NH4NO3(aq)
Solution
- Acid + Base b). Acid + Base
H2SO4 + NaOH HNO3 + NH3
Strong Strong Strong + Weak
∴ neutral solution ∴ acidic solution
4.9 Acid-base indicators
Indicators:
- can be used to determine whether a solution is acidic or basic;
- are weak acids that are in equilibrium with their conjugate bases (or vice versa) and
- have complex structures and formulae which will simply be represented as HIn (H followed by In for “indicator”).
NB: DEFINITION
- An indicator is a substance that changes colour in the presence of an acid or a base.
Indicator in equilibrium | |
| colour 1 | colour 2 |
Indicator in acid
| Indicator in base
|
e.g. Worked example 4
Bromothymol blue is an acid-base indicator. The colours it exhibits (shows) can be represented as follows:
- HIn ⇌ H+ + In−
yellow blue
A test tube contains a solution to which a drop of bromothymol blue has been added. The solution appears blue.
- What will you observe if a few drops of concentrated hydrochloric acid are added to the test tube?
- Explain your answer.
Solutions
- The colour of the solution changes from blue to yellow.
- Adding hydrochloric acid increases the concentration of the H+ ions in solution. According to Le Châtelier’s Principle the reverse reaction that opposes this change and decreases the [H+], will be favoured. Therefore the [HIn] increases and the colour changes to blue.
The most common indicators that are used in laboratories are:
INDICATOR | pH RANGE | COLOUR CHANGE |
methyl orange | 3,1 – 4,4 | red to orange to yellow |
methyl red | 4,2 – 6,2 | red to yellow |
litmus | 4,5 – 8,3 | red to blue |
bromothymol blue | 6,0 – 7,8 | yellow to blue |
phenolphthalein | 8.3 – 10 | pink-purple (magenta) in range, colourless outside range |
universal | 3-11 | red (3 and below); 3-6 orange/yellow; 7 green; 8-11 blue; 11 and above, violet. |
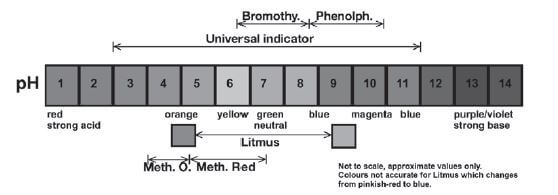
Choose the most suitable indicator for a particular titration. If you do not have Universal Indicator available, you should follow these steps:
Step by step – universal indicator
Step 1: Identify the strength of the acid and the base (pH range maximum and minimum)
Step 2: Draw a bracket to show the strength of the identified acid and base (shown as a line with arrowheads above)
Step 3: Note or mark the mid point (centre) of the bracket (or line as shown above)
Step 4: Use the indicator table to choose the indicator that shows the range of pH around the mid point (centre) of that bracket/line.
RESULT: You now have selected the appropriate indicator to use.
e.g. Worked example 5
Which one of the indicators given below will be the most suitable to be used in the titration of ethanoic acid against sodium hydroxide?
INDICATOR | pH COLOUR CHANGE RANGE | |
A | bromothymol blue | 6,0 – 7,8 |
B | phenolphthalein | 8,3 – 10 |
C | methyl orange | 3,1 – 4,4 |
D | methyl red | 4,2 – 6,2 |
Solution
Consult the table at Sections (3) and (4) above. We see Ethanoic Acid has a pH of about 2,4 (1M solution), and NaOH has a pH of about 14. The mid-point between 2,4 and 14 is about 5,8, so any indicator which changes from indicating acid to base around 5,8 would do. Answer: D; methyl red.
4.10 Acid-base titrations (volumetric analysis)
Titrations are used to experimentally determine the concentration of an unknown acid or base. When the titration results are used to determine the concentration of the unknown solution it is called a volumetric analysis.
Method and apparatus for an acid-base titration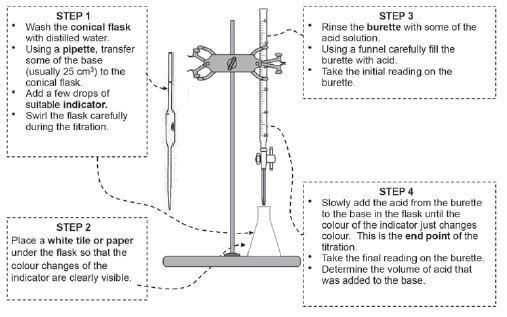
4.11 Preparing a standard solution
DEFINITION
A standard solution has a known concentration which remains constant for a period of time.
Standard solutions are often used in laboratories and it is important to know how to prepare a standard solution.
Concentration (c = n/V)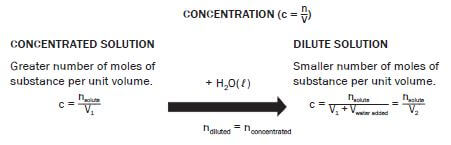
e.g. Worked example 6
hint: 1 cm3 = 1 ml ; 1 dm3 = 1 L
Aim: To prepare a 200 cm3 NaOH standard solution with a concentration of 0,5 mol∙dm–3.
Step 1: Calculate the mass of NaOH pellets required. M(NaOH) = 40 + 16 + 1 = 40 g·mol–1
Step 2: Use an electronic scale (balance) to measure off ± 4,4 g NaOH.
Note:
A specific mass of NaOH(s) can’t always be measured because it consists of pellets (not a fine powder).
Step 3: Note the exact mass of NaOH on the scale.
Step 4: Transfer the NaOH pellets to a volumetric flask. Add 100 cm3 of distilled water to the flask and seal it with a stopper. Shake the flask carefully until all the solute (NaOH) has dissolved.
Step 5: Slowly fill the flask to the calibration mark on the neck of the flask.
Step 6: Now calculate the exact concentration of the prepared solution – use the mass that was noted on the scale in Step 3. 
- n = m = 4,1 = 0,103 mol and c = n = 0,103 = 0,52 mol.dm-3
M 40 V 0,3
∴ a standard NaOH(aq) solution of concentration 0,52 mol·dm−3 has been prepared.
| Solute: | Solvent: | Solution: |
The substance (usually a solid) that is dissolved in the solvent. | The substance (usually distilled water) in which the solute is dissolved | The mixture of the solute and the solvent. |
 | ||
4.12 Dilution of solutions
We sometimes need to dilute a solution so that we can use it in a laboratory. We do this by taking a small amount of the solution and adding distilled water to it.
When diluting a solution, we need to know:
- the exact amount of distilled water that needs to added as well as
- the exact concentration of the dilute solution
In symbols: | c1 : concentration 1 (mol·dm–3) |
Remember:
- When a solution is diluted, the number of moles of substance remains constant.
- Only the volume of the solution changes (hence n is omitted in this equation.)
e.g. Worked example 7
Solution 1 has a concentration of 0,2 mol∙dm–3. Exactly 150 cm3 of solution A is transferred to a beaker 2 and 250 cm3 of distilled water is added to the beaker. Calculate the concentration of the diluted solution.
c1V1 = c2V2
(0,2)(0,15) = c2(0,4)
c2 = (0,2)(0,15)
0,4
= 0,075 mol.dm-3- The volume of the final solution (V2) is:
V2 = V1 = Vwater
=150 + 250 = 400cm3 - 400cm3
400 ÷ 1000 = 0,4 dm3 - convert to dm3
4.13 Acid-base titration calculations
In symbols:
- na = cava
nb cbvb - c = n
V
where OR c = m
MV
n = m
M- n : mol (mol)
- c : concentration (mol·dm–3)
- V : volume (dm–3)
- a : acid
- b : base
e.g. Worked example 8
During a titration 20 cm3 diluted H2SO4 precisely neutralises 25 cm3 of a NaOH solution. If the concentration of the H2SO4 solution is 0,5 mol∙dm–3, calculate the concentration of the NaOH.
Solution
First write down the balanced reaction.
1 H2SO4(aq) + 2 NaOH(aq) → Na2SO4(aq) + H2O(ℓ)
na = 1
nb = 2
Va = 20 cm3
Vb = 25 cm3
ca = 0,5 mol∙dm–3
cb = ?
Find the mol ratio of
- acid : base
H2SO4 : NaOH
1 : 2 - na = cava
nb cbvb - 1 = (0,5)(20)
2 cb(25) - 1.cb (25) = (0,5)(20)(2)
- cb = 0,8 mol∙dm–3
e.g. Worked example 9
TITRATION CALCULATIONS
Eight grams (8,0 g) of sodium hydroxide are dissolved in 350 cm3 of distilled water. 15 cm3 of this solution neutralises 20 cm3 of a sulphuric acid solution. The balanced equation for this reaction is:
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(ℓ)
Calculate the concentration of the sulphuric acid solution.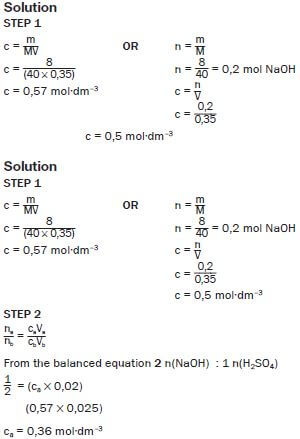
e.g. Worked example 10
The titration of oxalic acid with sodium hydroxide
Part 1:
Aim: To prepare a 250 cm3 of oxalic acid standard solution with a concentration of 0,08 mol∙dm–3
Solutions
Step 1
- Measure between 2,5 and 2,7 g of pure oxalic acid hydrate crystals (COOH)2 · 2H2O into the glass beaker and weigh again.
- Add 30-60 cm3 of distilled water to the glass beaker and dissolve the crystals.
- Transfer the solution into a clean 250 cm3 volumetric flask.
- Rinse the beaker with 15-20 cm3 of distilled water and pour this solution into the volumetric flask and repeat. This will ensure that all of the oxalic acid is transferred into the volumetric flask.
- Fill the volumetric flask to within about 2 cm of the mark and allow it to sit for a minute. This will allow any water clinging to the edges of the neck to drain into the flask. Using an eyedropper, fill the flask to the calibrated mark with water.
- Stopper (close) the flask and mix the solution by repeated inversion (turning upside-down) and swirling. This requires about 30 inversions and takes close to 1 minute.
Step 2
- Calculate the exact concentration of the oxalic acid solution using the mass of acid used and the volume of the volumetric flask and record the concentration of your solution on the bottle.
If you have used the following
- Mass oxalic acid crystals 2,6 g
- Final volume solution 250,0 cm3
Using the above information to calculate the number of moles and concentration of oxalic acid crystals.
Part 2:
Procedure to perform (to do) a titration of sodium hydroxide with oxalic acid.
Aim: use a standard solution of oxalic acid to determine the concentration of sodium hydroxide
H2C2O4(aq) + 2 NaOH(aq) → 2 H2O(ℓ) + Na2C2O4(aq)
Solutions
- Measure exact 25,00 cm3 of the oxalic acid standard solution (c = 0,084 mol·dm–3) into a flask and add few drops of phenolphthalein.
- In this titration the oxalic acid solution is acidic and therefore phenolphthalein will be colourless.
- The sodium hydroxide solution will be added drop wise (drop by drop) from a burette into the flask containing the oxalic acid and indicator.
- As the sodium hydroxide is added to the flask it will react with the oxalic acid and be neutralized.
- At the point where all of the oxalic acid is reacted, the next drop of sodium hydroxide will make the entire solution basic and it will turn pink. At this point you have completed the titration.
Results (If the following data and results were obtained after at least three trials)
- Volume oxalic acid solution 25,00 cm3
- Volume sodium hydroxide to titrate 19,43 cm3
Mole of sodium hydroxide reacted:
- na = cava
nb cbvb
First write down the balanced reaction.
- 1 H2C2O4(aq) + 2 NaOH(aq) → 2 H2O(ℓ) + Na2C2O4(aq)
Find the mol ratio of acid : base ... ratio of H2C2O4 : NaOH = 1 : 2
- na = 1
- nb = 2
- Va = 20 cm3
- Vb = 25 cm3
- ca = 0,5 mol·dm–3
- cb = ?
na = cava
nb cbvb
1 = (0,084)(25)
2 cb(25)
1· cb (19,43) = (0,084)(25)(2)
cb = 0,216 mol·dm–3
Part 3:
In order to determine the concentration of acetic acid in the vinegar solution you will titrate it with the standardized sodium hydroxide solution. The equation for this reaction is
1 CH3COOH(aq) + 1 NaOH(aq) → H2O(ℓ) + CH3COONa(aq)
The following data was obtained during titration
Data
- Volume vinegar solution 25,00 cm3
- Volume sodium hydroxide titrated 22,84 cm3
Calculations for determining concentration of acetic acid in vinegar First write down the balanced reaction.
1 CH3COOH(aq) + 1 NaOH(aq) → H2O(ℓ) + CH3COONa(aq)
Find the mol ratio of acid : base ... CH3COOH : NaOH = 1 : 1
From a balanced equation 2 n(NaOH) : 1 n(H2SO4)
In order to get the best precision possible, you should repeat each titration until you get 3 trials that are within 1% of each other.
4.14 The Equilibrium Constant (Kc) (The Law of Mass Action)
NB
Solids and pure liquids are omitted from the Kc expression as their concentration is [1], as multiplying by 1 has no effect.
The concentrations of all the compounds (solutions and gases) in a closed system in dynamic chemical equilibrium are related by a mathematical equation. The numerical value of this equation is called the equilibrium constant (Kc).
In the hypothetical equation below the equilibrium expression for this reaction is:
- 2P + 3R ⇋ 2S + 4T
Kc = [S]2 × [T]4
[P]2 × [R]3
hint
Kc: equilibrium constant (no unit)
[substance]: concentration of reactant or product (in mol·dm–3) mol: number of moles of each compound in the balanced reaction equation.
NB
- This is the correct way of writing the Kc expression
- The coefficients are the moles of each reactant and product in the balanced equation
- The product of the concentration of reactants (not to be added, but multiplied!!), raised to the power of the number of moles is the numerator
- The product of the concentration of products (not to be added, but multiplied!!), raised to the power of the number of moles is the denominator
- The concentrations used in the Law of Mass Action is the [reactant]equilibrium and [product]equilibrium (NOT initial concentrations!! i.e. the concentrations of reactants and products at equilibrium)
4.15 Ka and Kb values
Since the ionisation of a weak acid is an equilibrium, a chemical equation and an equilibrium constant expression can be written as follows. Remember that square brackets means “concentration”, or moles per dm3.
- HA + H2O ⇋ H3O+ + A– K = [H3O+][A–]
[HA]
The equilibrium constant for the ionisation of an acid is called the acid ionisation constant (Ka).
A similar expression can be written for bases:
- A + H2O ⇋ HA+ + OH- Kb = [OH–][HA+]
[A–]
The equilibrium constant for the ionisation of a base is called the base ionisation constant (Kb)
NB
An acid or base’s strength refers to its degree of ionisation. A strong acid will completely ionise in water while a weak acid will only partially ionise. Since there are different degrees of ionisation, there are different levels of weakness. Fortunately, there is a simple quantitative way of expressing this.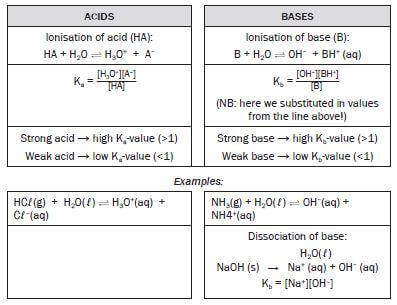
Activity 6
Do you think a strong acid will have larger or smaller Ka value? Explain your answer. (3) [3]
Solution
- The strong acid will have a larger Ka value.
- A strong acid is a better proton donor, resulting in more products.
- Since the concentration of the products is in the numerator of the Ka expression, the stronger the acid, the larger the Ka. [3]
Activity 7
Choose the strongest base in the list below by comparing their Kb values.
Base Kb
- Ammonia, NH3 1,8 × 10–5
- Hydroxylamine, HONH2 9,1 × 10–9
- Ethylamine, C2H5NH2 4,3 × 10–4 (2) [2]
Solution
- C [The larger the Kb, the stronger the base] [2]
Solids and pure liquids are NEVER written in the equations for the equilibrium constant Kc, or Ka or Kb as their concentration is [1]
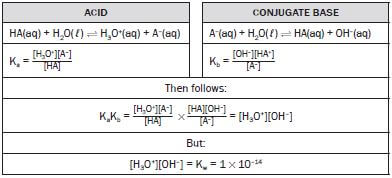
4.16 The relationship between Ka and Kb for a substance
We know that the strength of a conjugate base is inversely proportional to the strength of the conjugate acid; i.e. weak acids produce strong conjugate bases, and vice versa.
NB
You are aware that the pH scale runs from 1 to 14... now look at the values above again.
4.17 Auto-ionisation of water
Water is an ampholyte and can react as an acid (donates a H+) or a base (accepts a H+).
Two water molecules can undergo autoprotolysis or auto-ionisation. One water molecule can act as an acid and donate a H+ to the other water molecule, the base.
An acid : H2O ⇋ H+ + OH−
A base : H2O + H+ ⇋ H3O+
- Water is a weak acid and a weak base.
- Low % auto-ionisation occurs.
- Pure water is a weak electrical conductor.
Net reaction showing the conjugate acid-base pairs: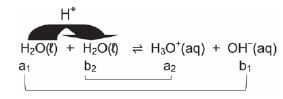
4.18 Equilibrium constant for water (Kw)
From the acid-base reaction for water the equilibrium expression for water can be written.
H2O(ℓ) + H2O(ℓ) ⇋ H3O+(aq) + OH−(aq)
- Kc = [H3O+][OH−]
- Kw = [H3O+][OH−]
Kw : equilibrium constant for water
At 25°C: Kw = 1 × 10–14
4.19 The pH scale
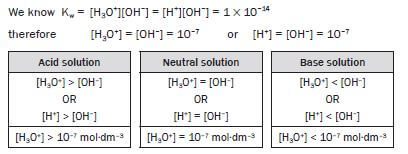
e.g. Worked example 11
Determine whether the following solutions are acidic, basic or neutral:
| A solution with |
|
| [H3O+] = 10−3 mol·dm–3 [H3O+] = 10−12 mol·dm–3 |
NB: DEFINITION
- pH of a solution is the negative logarithm of the hydronium ion concentration in a solution. pH = log [H3O+]
It is complicated to continually refer to the hydronium ion concentration. The pH scale is therefore used as a simplified method of indicating the degree of acidity of a solution.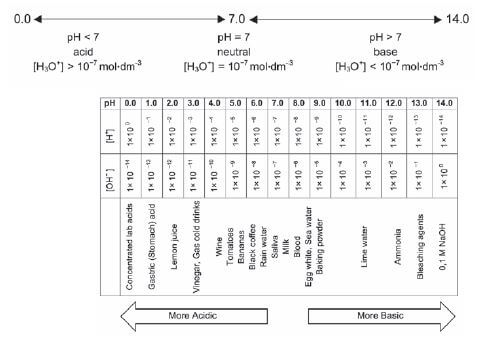
4.20 pH Calculations for strong acids and bases
To calculate the pH of a strong acid or base, these steps should be followed:
Acid | Step | Base |
0,1 mol·dm–3 of HCℓ solution | For example: | 0,5 mol·dm–3 of NaOH solution |
| Write down the ionisation reaction for the acid. HCℓ + H2O ⇋ 1 H3O+ + Cℓ− | 1 | Write down the dissociation reaction for the base. 1NaOH(s) ⇋ Na+(aq)+OH− (aq) |
Find the mol ratio of acid to hydronium ions | 2 | Find the mol ratio of base to hydroxyl ions 1 mol NaOH : 1 mol OH− |
| Determine the [H3O+] [HCℓ] = 0,1 mol·dm–3 [H3O+] = 0,1 mol·dm–3 | 3 | Determine the [OH–] |
| No step 4 for acids | 4 | Determine the [H3O+] |
Calculate the pH | 5 | Calculate the pH |
e.g. Worked example 12
Calculate the pH of a H2SO4 solution of concentration 0,6 mol∙dm–3.
Solutions
Step 1: 1 H2SO4(g) + 2 H2O(ℓ) ⇋ 2 H3O+(aq) + SO42−(aq)
Step 2: 1 mol HCℓ : 2 mol H3O+
Step 3: [H2SO4] = 0,6 mol·dm–3 ∴ [H3O+] = 2(0,6) = 1,2 mol·dm–3
Step 4: pH = − log [H3O+]
− log 1,2
0,08
4.21 Summary: Strong and weak acids and bases
Acids ionise in water to form ions:
Examples:
HCℓ(g) + H2O(ℓ) ⇋ H3O+(aq) + Cℓ−(aq)
CH3COOH(g) + H2O(ℓ) ⇋ H3O+(aq) + CH3COO–(aq)
| STRONG AND WEAK ACIDS | |
| STRONG ACIDS | WEAK ACIDS |
High % ionisation | Low % ionisation |
Examples: | Examples: |
| Strong acid ⇌ weak conjugate base + H+ HCℓ ⇌ Cℓ−+H+ | Weak acid ⇌ strong conjugate base + H+ CH3COOH ⇌ CH3COO− + H+ |
Remember:
- The electrical conductivity of any electrolyte is determined by the number of ions in solution. The greater the ion concentration, the higher the conductivity.
- Reaction rate is determined by the concentration of a reactant. The higher the concentration of the ions in a solution, the higher the rate of reaction.
| STRONG AND WEAK BASES | |
| STRONG BASES | WEAK BASES |
High % dissociation | Low % dissociation or ionisation |
| NaOH Sodium hydroxide KOH Potassium hydroxide | NH3 Ammonia |
| strong base + H+ ⇌ weak conjugate acid OH− + H+ ⇌ H2O | weak base + H+ ⇌ strong conjugate acid |
Remember:
- The pH of an acid is dependent on the relative concentrations of the H3O+ and OH− ions in the solution.
| In general: | |||||
pH 1 - 2 | : | strong acid | pH 12 - 14 | : | strong base |
pH 3 - 6 | : | weak acid | pH 8 - 11 | : | weak base |
| pH 7 | neutral | ||||
4.22 Summary: Concentrated and dilute acids and bases
Concentration of an acid or a base is an indication of the number of moles of solute per unit volume. Both strong and weak acids and bases can be either concentrated or diluted.
Examples: | |
1 mol·dm–3 | HCℓ(aq) : concentrated solution of a strong acid |
0,01 mol·dm–3 | HCℓ(aq) : diluted solution of a strong acid |
1 mol·dm–3 | CH3COOH(aq) : concentrated solution of a weak acid |
0,01 mol·dm–3 | CH3COOH(aq) : diluted solution of a weak acid |
1 mol·dm–3 | NaOH(aq) : concentrated solution of a strong base |
0,01 mol·dm–3 | NaOH(aq) : diluted solution of a strong base |
1 mol·dm–3 | NH3(aq) : concentrated solution of a weak base |
0,01 mol·dm–3 | NH3(aq) : diluted solution of a weak base |
Activity 8
- A standard solution is a solution ...
- at 25˚C
- of an acid or a base
- of which the volume is known
- of which the concentration is known (2)
- Consider the following ionisation equilibrium:
H2O(ℓ) ⇋ H+(aq) + OH−(aq)
The ionisation constant of water (Kw) in the above reaction increases from 1 × 10–14 at 25˚C to 9,6 × 10–14 at 60˚C. Which one of the following statements is therefore correct- [H+] > [OH−] at 60˚C
- The ionisation reaction is exothermic
- The pH increases with an increase in temperature
- The pH decreases with an increase in temperature (2)
- Consider the reaction:
CH3COOH(g) + H2O(ℓ) ⇋ H3O+(aq) + CH3COO–(aq)
The ionisation constant for this reaction at 25°C is 1,8 × 10–5. The equilibrium constant for this reaction at 60°C, is 3,6 × 10–7. From this information we can deduce that forward reaction ...
(Explain your choice).- is endothermic
- is exothermic
- is a redox reaction
- is a precipitation reaction (2)
- Two beakers, A and B, contain solutions of the same concentration with a pH of 2 and 4,5 respectively. Which of the following combinations is correct?
Beaker A Beaker B- Weak Acid Strong Acid
- Strong Acid Weak Acid
- Strong base Weak base
- Weak base Strong base (2)
- Andile rinses the apparatus before starting an acid-base titration experiment. Which rinsing method is likely to cause inaccurate results?
- The Erlenmeyer (conical) flask is rinsed with distilled water
- The burette is rinsed with the acids it is to be filled with
- The pipette is rinsed with the base it is to be used for
- The volumetric (measuring) flask, which is used to make up the standard solution of the base, is rinsed with distilled water. (2)
- If base X is titrated against acid Y, the pH of the solution at the end point is 8. The base X and acid Y are respectively:
X Y- NaOH CH3COOH
- Na2CO3 HCℓ
- NaOH H2SO4
- Na2CO3 CH3COOH (2) [12]
Solutions
- D ✓✓(2)
- D
 (2)
(2) - B✓✓ Kc at 25°C is greater than Kc at 60°C
Kc = [CH3COO–][ H3O+]. If the mixture is heated, the Kc decreases (2) - B✓✓ Beaker A has a low pH which indicates that it contains more H+, therefore it has ionised completely. Whilst beaker B has a high pH which indicates few H+ ions, thus it has partially ionised. (2)
- A✓✓ The Erlenmeyer (conical) flask must be rinsed with distilled water. Only the number of moles of base which was measured in the pipette must be neutralised. (2)
- A✓✓ (2) [12]
Activity 9
- Write down:
1.1 The meaning of the term diprotic acid. (2)
1.2 The formula of a diprotic acid. (1) - Magnesium hydroxide (Mg(OH)2) is often used as medicine to relieve an upset stomach. The pH of the HCℓ(aq) in a person’s stomach is 1.
2.1 Calculate the concentration of the hydrochloric acid in the person’s stomach. (3)
2.2 Will the pH in the stomach INCREASE, DECREASE or STAY THE SAME after taking in a dose of Mg(OH)2? (2)
2.3 A person takes in a dose of Mg(OH)2. Write down the balanced equation for the reaction that takes place in the stomach. (3) - A textbook states that calcium sulphate (CaSO4) is slightly soluble in water. Two learners decided to test the dam water from a local municipality for calcium sulphate. They took a 0,5 dm3 sample of the dam water and treated it with sodium carbonate solution to precipitate the calcium ions present according to the following equation:
CaSO4(aq) + Na2CO3(aq) → Na2SO4(aq) + CaCO3(s)
The precipitate is then dissolved in 30 cm3 of 0,1 mol·dm–3 HCℓ solution which converts the precipitate to aqueous calcium chloride, water and carbon dioxide according to the following equation:
CaCO3 + 2HCℓ → CaCℓ2 + CO2 + H2O
The HCℓ was in excess. They neutralised the excess HCℓ by adding 15,8 cm3 of a 0,1 mol·dm–3 NaOH solution. The equation for the reaction is:
HCℓ + NaOH → NaCℓ + H2O
Calculate the mass of calcium sulphate that was present in the sample of dam water. (10) [21]