THE CHLOR-ALKALI INDUSTRY GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
Share via Whatsapp Join our WhatsApp Group Join our Telegram Group- Summary
- Definitions and terminology
- The chlor-alkali industry – reactants and products
- The chlor-alkali industry – industrial process
The chlor-alkali industry
The chlor-alkali industry is a large worldwide multimillion industry which produces compounds that are essential to human existence. The industry is based on the electrolysis of sodium chloride solutions.
6.1 Summary
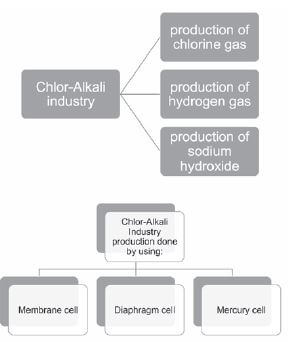
Chlor-Alkali industry involves the electrolysis of a sodium chloride solution which produces:
- chlorine gas;
- hydrogen gas and
- sodium hydroxide (aq).
6.2 Definitions and terminology
The processes involved are electrolysis, oxidation and reduction.
Electrolysis:
- is an endothermic reaction
- involves a process during which electric energy is converted to chemical energy or brings about a chemical change
- involves a non-spontaneous redox reaction.
Oxidation:
- takes place when electrons are given off
- at the anode.
Reduction:
- takes place when electrons are received
- at the cathode.
Terminology:
- Corrosive: Substance that damages and destroys substances (including living matter) it comes into contact with (touches).
- Leaching: Movement of a substance (like NaOH (aq), pesticides or fertilisers) that are carried by water, down through soil (ground) to the underground water table. The water table is the level below which the ground is saturated with water.
- Carbon footprint: The amount of carbon dioxide and other greenhouse gases released into the atmosphere due to the activities of a person, organisation or process.
- Brine: A concentrated solution of NaCℓ(aq)

You must know how to:- Identify the processes involved
- Give a description of the concept or term
- Give the correct definition
- Describe the process and chemicals involved/ produced
- State uses of the products
- State the environmental issues
6.3 The Chlor-alkali industry – reactants and products
| REACTANTS | |||
| |||
PRODUCTS | |||
| Chlorine gas Cℓ2(g) | Hydrogen gas H2(g) | Sodium hydroxide (caustic soda) NaOH(aq) | |
|
|
| |
| USES | Reactant for production of:
Used to:
| Reactant for production of:
| Reactant for production of:
Used to:
|
NB: REMEMBER:
The membrane cell is preferred because the:
- components of the cell are non-toxic;
- products are more pure and
- production costs are relatively low.
- Sodium chloride (table sale) NaCℓ(s) is an ionic compound and is very soluble in water (a polar solvent).
- NaCℓ (s) dissociates in an aqueous solution – the ions in the ionic crystal lattice separate.
- NaCℓ(s) H2O (ℓ) Na+ (aq) + Cℓ– (aq)
- A sodium chloride solution, NaCℓ (aq), is known as brine.
6.4 The chlor-alkali industry – industrial process
The chlor-alkali industry products can be produced by using a:
- membrane cell
- mercury cell
- diaphragm cell
| Membrane Cell | Diaphragm Cell | Mercury Cell | |
| Purity and yield of Cℓ2 (g) produced |
|
|
|
| Purity of NaOH (aq) produced | High |
| Low |
| Energy consumption (Affects costs) | Lowest | Relatively low | Very high |
| Environmental impact |
|
|
|
| Health risks |
|
|
|
NB:
- Very few companies still use mercury cells but many still use diaphragm cells. When new industrial sites are set up today, membrane cells are mostly used.
e.g. Worked example 1
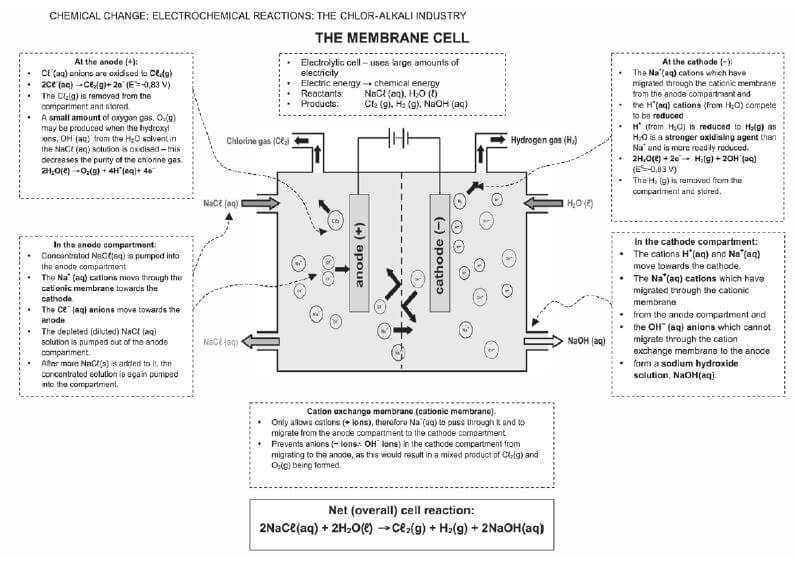
1. Chlorine is produced industrially by electrolysis in an electrolytic cell and can be represented as follows:
2NaCℓ(aq) + 2H2O(ℓ) → H2(g) + Cℓ2(g) + 2NaOH(aq)
| I | 2H2O | + | 2e– | → | H2 | + | 2OH– |
| II | Cℓ2 | + | 2e– | → | 2Cℓ– | ||
| III | 2Cℓ– | → | Cℓ2 | + | 2e– | ||
| IV | H2 | + | 2OH– | → | 2H2O | + | 2e– |
The correct statement(s) is/are:
- I and III
- I only
- II and IV
- III only
2. Chlorine is a poisonous gas commonly used as a bleaching agent. Chlorine is produced in industry by _____________.
Solutions
- A
- Electrolysis of brine
Activity 1
The electrolysis of saturated sodium chloride can be illustrated as follows:
Hydrogen and chlorine bubble off at the electrodes.
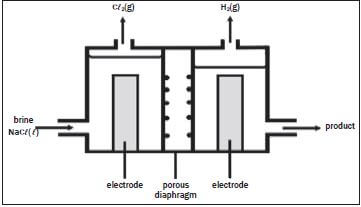
- Give an equation to show how chlorine bubbles are formed at the electrode. (2)
- At which electrode are the chlorine bubbles formed? (1)
- At which electrode does hydrogen gas form? (1)
- What is the name of the product which leaves the cell? (1)
- Give TWO applications of the product formed in 2.4. (2)
- What purpose does the porous diaphragm serve? (2)
- The chlorine gas produced, dissolves in water to form chlorine water. Write down a balanced equation for the reaction that takes place. (2) [11]
Solutions
- 2Cℓ–(aq) → Cℓ2(g) + 2e– (2)
- Anode (1)
- Cathode (1)
- Sodium hydroxide (1)
- Making soap and detergents; paper; rayon and other fibres; dyeing textiles (any 2) (2)
- Stops chlorine passing through; helps to separate sodium hydroxide from NaCℓ(aq). (2)
- Cℓ2 + H2O → HCℓ + HOCℓ (2) [11]
e.g. Worked example 2
The diagram below shows a type of membrane cell used in the chlor-alkali industry.
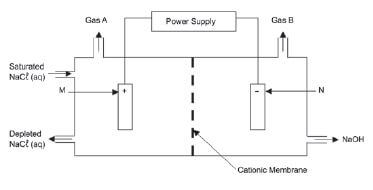
- Name the gases A and B
- Why is the membrane called a cationic membrane?
- Write down the half-reaction that takes place at electrode N.
- Apart from its use in household products, name ONE industrial use of chlorine.
- Explain why this electrolytic process cannot be done in one large container without a membrane.
Solutions
- A: Chlorine
B: Hydrogen - Allows only the positive ions (cations) to pass through it.
- 2H2O + 2e– → H2 + 2OH–
- Manufacture of PVC, paper, drugs, etc
Disinfectant for water - In a single pot the chlorine will react with water to form chlorine water/or the chlorine will react with the OH– ions to form bleach OR the products formed will be contaminated
Activity 2
The simplified diagram of a cell used in the chlor-alkali industry is shown below.
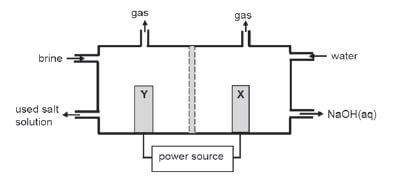
- Write down the CHEMICAL FORMULA of brine. (1)
- At which electrode, X or Y is chlorine gas formed? (1)
- Write down a half-reaction that explains the formation of hydrogen gas at one of the electrodes. (4)
- The purity of the sodium hydroxide produced in the chlor-alkali industry depends on the extent to which it is separated from the chlorine gas produced by this cell. Briefly describe how chlorine gas and sodium hydroxide are prevented from mixing in this cell. (2)
- Apart from the advantages and disadvantages of products produced, write down for this process:
5.1 ONE positive impact on humans. (1)
5.2 ONE negative impact on humans (1) [10]
Solutions
- NaCℓ (aq) (1)
- Y (1)
- 2H2O + 2e– →H2+2OH – (4)
- The membrane prevents chloride ions from moving to the cathode, only allows positive ions through. (2)
- 5.1 Job creation resulting in more people having a better life. (1)
5.2 Uses huge amounts of electricity resulting in load shedding.
OR
Chemical plant uses a lot of space that could have been used for housing/gardens, etc. (any one) (1)
