THE FERTILISER INDUSTRY GRADE 12 NOTES - PHYSICAL SCIENCE PAPER 2: CHEMISTRY STUDY GUIDES
Share via Whatsapp Join our WhatsApp Group Join our Telegram Group- Nutrients required by plants
- Functions and sources of the primary mineral nutrients
- The industrial production of fertilisers
- Flowchart: The industrial production of fertilisers
- N:P:K fertilisers
- Excessive use of fertiliser and the environment
- Alternatives to inorganic fertilisers
The fertiliser industry
The need for fertilisers is increasing due to:
- the growth in the world population – more people means more food is necessary and
- the decrease in available agricultural land – less land means the available land must produce a lot of high quality food at a high rate (fast).
Nutrients in the soil are used by the plants that grow and these nutrients must be replaced.
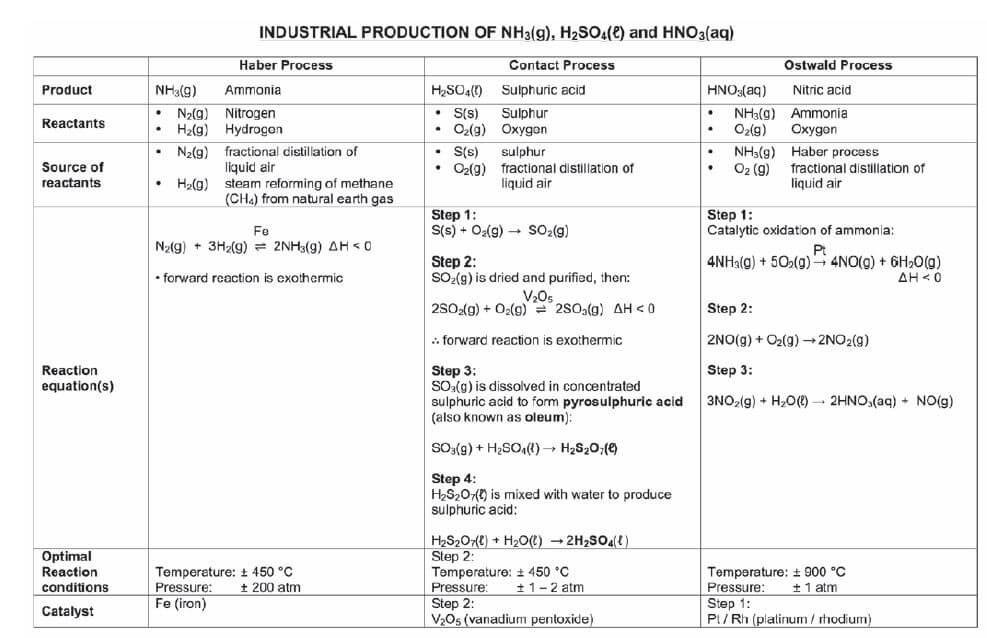
Inorganic fertilisers are produced industrially in the Haber, Contact & Ostwald processes at chemical plants like SASOL.
7.1 Nutrients required by plants
| MACRO NUTRIENTS | MICRONUTRIENTS | |||
Primary non- mineral nutrients | Primary mineral nutrients | Secondary nutrients | Trace elements | |
| ELEMENTS |
|
|
|
|
| SOURCES |
|
|
|
|
| AVAILABILITY |
|
|
|
|
NB: DEFINITIONS
- Promote: to help or encourage
- Essential: absolutely necessary
- Inorganic: not produced by a living organism; not containing carbon
- Mineral: an inorganic solid substance that occurs naturally (not man-made)
- Inert: unreactive
- Sufficient: enough
Did you know?
- Bone meal is produced when the bones of dead animals are ground into powder.
- Kelp meal is dried seaweed.
- Granite meal is finely ground granite rock.
Activity 1
- Which of the following is a primary mineral nutrient that is needed by plants?
- N
- C
- Mg
- Na (2) [2]
Solution
1. A [2]
7.2 Functions and sources of the primary mineral nutrients
| PRIMARY MINERAL NUTRIENTS | |||
| Nutrient | (N) | Phosphorous (P) | Potassium (K) |
| Function |
|
|
|
| Sources Pre- World War II |
|
|
|
|
|
| |
|
|
| |
Atmospheric nitrogen is fixed into compounds containing nitrates (NO3−) or ammonium ions (NH4+) so that the nitrogen can be taken in by plants and used as nutrient.
- Atmospheric fixation: fixation by lightning;
- Biological fixation: fixation by bacteria in the ground and by the roots of legumes;
- Industrial fixation: fixation by industrial processes like the Haber process.
hint
- “Fixed” means “attached”.
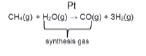
- In the Haber process:
Steam reforming of methane (natural gas) in the presence of a platinum catalyst to form a mixture of carbon monoxide (CO) and hydrogen (H2) gases (synthesis gas).
- In the Contact process:
2.1 If SO2(g) is not dried before step 2, it reacts with water to form sulphurous acid, H2SO3(aq) which forms acid rain, a type of rain which is produced as a side-effect of human industrial activities, which is corrosive to structures and harmful to living things.
SO2(g) + H2O(ℓ) → H2SO3(aq)
2.2 SO3(g) reacts with water to form gaseous sulphuric acid, H2SO4(g) which escapes into the atmosphere and forms acid rain.
SO3(g) + H2O(ℓ) → H2SO4(g) - In the Contact process:
3.1 The 1st step is known as the catalytic oxidation of ammonia.
Pt
4NH3(g) + 5O2(ℓ) → 4NO(g) + 6H2O(g)
3.2 NO2 (nitrogen dioxide) is a brown gas which reacts with water to form HNO3(g) which escapes into the atmosphere and forms acid rain.
Fluorapatite, Ca5(PO4)3F
- is mined at Phalaborwa;
- is insoluble in water and can’t be absorbed by plant roots;
- reacts with sulphuric acid, H2SO4(ℓ) to produce phosphoric acid, H3PO4(aq).
Superphosphate
- is produced when fluorapatite reacts with sulphuric acid H2SO4 and is a mixture of Ca(H2PO4)2·H2O and CaSO4 (gypsum)
Triple Superphosphate
- is produced when fluorapatite reacts with phosphoric acid H3PO4 to form Ca(H2PO4)2·H2O (no CaSO4 is formed).
7.3 The industrial production of fertilisers
| Organic | |||||
| Fertiliser | Ammonium sulphate | Ammonium nitrate | Monoammonium phosphate (MAP)
| Superphosphate
| Urea (NH2)2CO |
| Reactants | NH3(g) H2SO4(ℓ) | NH3(g) Ammonia HNO3(aq) | NH3(g) Ammonia H3PO4(aq) | Ca5(PO4)3F fluorapatite H2SO4 | NH3(g) Ammonia CO2(g) |
| Reaction equation | 2NH3(g) + H2SO4(ℓ) → (NH4)2SO4(aq) | NH3(g) + HNO3(aq) → NH4NO3(aq) | NH3(g) + H3PO4(aq) → NH4H2PO4(aq) 2NH3(g) + H3PO4(aq) → (NH4)2HPO4(aq) | Not required | 2NH3(g) + CO2(g) → (NH2)2CO(g) + H2O(g) |
| Properties |
|
|
|
|
|
7.4 Flowchart: The industrial production of fertilisers
e.g. Worked example 1
Study the diagram below that illustrates the industrial preparation of nitric acid and answer the questions that follow.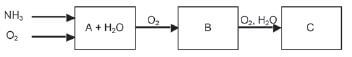
- 1.1 Write down a balanced chemical equation for the formation of product A.
1.2 Name the catalyst used during the reaction in Question 2.1.
1.3 What name is given to the reaction in Question 2.1? Give a reason for this name.
1.4 Write down a balanced chemical equation for the formation of product B.
1.5 Name the products A and B.
1.6 Write down a balanced chemical equation for the formation of product C.
1.7 Name product C. - Ammonium nitrate is important for its use in fertilisers and explosives. It can be prepared by the reaction of nitric acid and ammonia.
2.1 Write down a balanced equation for the preparation of ammonium nitrate.
2.2 What kind of fertiliser is ammonium nitrate?
2.3 Which characteristic of ammonium nitrate makes it suitable for use as a fertiliser?
2.4 Which characteristic of ammonium nitrate makes it suitable to be used in explosives? - Ammonium sulphate is often used as a fertiliser to supplement nitrogen and sulphur shortages in plants.
3.1 Compile a flow chart that indicates all the industrial steps for the preparation of ammonium sulphate from air, natural gas and sulphur.
3.2 Give balanced equations for all the reactions that take place during the preparation of ammonium sulphate from air, methane and sulphur.
Solutions
- 1.1 Pt
4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
1.2 Platinum or Rhodium
1.3 Catalytic oxidation of ammonia
1.4 2NO(g) + O2(g) → 2NO2(g)
1.5 A: Nitrogen monoxide B: Nitrogen dioxide
1.6 3NO2(g) + H2O(ℓ) → 2HNO3(aq) + NO(g)
1.7 Nitric Acid - 2.1 HNO3 + NH3 → NH4NO3
2.2 Primary mineral nutrient
2.3 High nitrogen content per mass
2.4 Nitrogen bonds are easily broken and therefore it decomposes rapidly and easily (explodes). - Answers for 3.1 and 3.2
- O2 + N2 (air)
- CH4 (methane)
- S
- To produce: (NH4)2SO4 (ammonium sulphate)
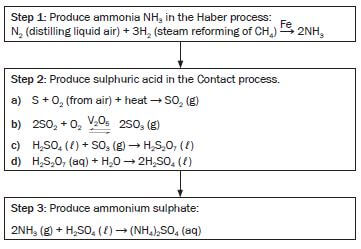
7.5 N:P:K fertilisers
As plants need a large amount of the primary mineral nutrients (nitrogen, phosphorus and potassium) and these need to be replenished (replaced) in the soil to make sure that crops grow well to provide enough food, NPK fertilisers that contain a mix of these nutrients, are usually used. They are produced by mixing a nitrogen fertiliser, a phosphorus fertiliser and potassium chloride (KCℓ).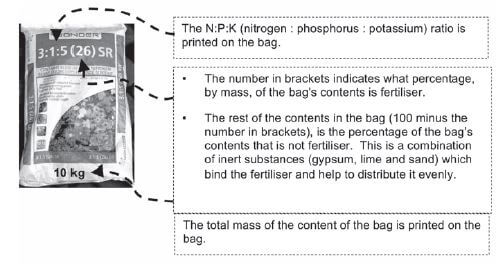
Step by step
Step 1. Find the mass of the bag.
Step 2. Find the percentage (%) of fertiliser in the bag (the number in brackets).
- 26% fertiliser in bag
∴ 74% inert compounds
Step 3. Find the mass of fertiliser in the bag.
- mass of fertiliser = percentage fertiliser × mass of bag
100
= 26 × 10Kg = 2,6 Kg
100
Step 4. Find the total number of parts of nutrients in the bag. (Add the numbers representing the ratios of each of the elements, N, P and K.)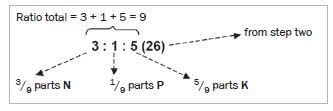
Step 5. For each element (N, P and K) find the percentage of the element in the fertiliser:
- % element = ratio element × % fertiliser
- %N= ratio N ×% fertiliser = 3 × 26 = 8,67
total ratio 9 - %P= ratio P ×% fertiliser = 1 × 26 = 2,89
total ratio 9 - %P= ratio K ×% fertiliser = 5 × 26 = 14,44
total ratio 9
Step 6. For each element (N, P and K) find the mass of that element in the bag.
- mass element = ratio element × % fertiliser × mass of bag
total ratio 100- 3 × 26 × 10 kg = 0,87 kg
9 100 - 1 × 26 × 10 kg = 0,29 kg
9 100 - 5 × 26 × 10 kg = 1,44 kg
9 100
- 3 × 26 × 10 kg = 0,87 kg
The per component totals (0,87; 0,29; 1,44) add up to 2.6kg.
e.g. Worked example 2
A bag of fertiliser has the following information on it: 3 : 2 : 3 (26).
- What information can you deduce from these numbers?
- Calculate the percentage composition of the fertiliser in the bag.
Solutions
- It shows the ratio N : P : K.
3 + 2 + 3 = 8therefore the fertiliser mixture consists of:
3/8 parts N; 2/8 parts P and 3/8 parts K
These nutrients make up 26% of the mass of the bag content. The other 74% is made up of gypsum, lime and sand. - %N= ratio N ×% fertiliser
total ratio- =3 × 26 = 9,75
8 - = 2 × 26 = 6,5
8 - = 3 × 26 = 9,75
8
- =3 × 26 = 9,75
7.6 Excessive use of fertiliser and the environment
The correct application of fertiliser to crops is essential for high quality, fast growing crops but using too much or unnecessary fertiliser has a negative effect on the environment.
- Groundwater is contaminated by fertiliser leaching (spreading by water) into the ground.
- Soil becomes acidic (pH decreases). Many plants do not grow in acidic soil.
- Invasive plants grow excessively while indigenous plants die. Invasive plants are undesirable non-indigenous (foreign) plants that grow too fast and out-compete local plants.
- Fertiliser in dams and rivers leads to “eutrophication”, defined below.
- High nitrate concentrations in drinking water decreases the ability of haemoglobin in the blood to carry oxygen and leads to ‘blue baby syndrome’.
7.6.1 Eutrophication
| In water (dams and rivers) | On land | |
| Causes |
| |
|
| |
| Effexts |
|
|
| Possible solutions |
|
|
NB: REMEMBER
An overabundance (excess, too much) of nutrients (N and P) in water leads to:
- the fast and excessive growth of algae and other water plants (this is called ‘algal bloom’) and bacteria
- causing a drop in the oxygen concentration in the water and
- the death of fish and other living organisms in the water.
hint: VOCABULARY
- Overabundance / excess: more than is needed
- Shortage: not enough
- Invasive plants: plants that do not naturally grow in a certain area
- Indigenous plants: plants that grow naturally in (are native to) a certain area
- Swamps: ground that is uncultivated but is usually covered with water
- Erosion: soil and rock is removed from the Earth’s surface by wind, rain or water
- Stunted growth: reduced growth leading to smaller, underdeveloped plants
Activity 2
- Which ONE of the following is NOT associated with eutrophication in water?
- Dead zones
- Algal bloom
- Depletion of oxygen
- Increased aquatic life (2) [2]
Solution
- D [2]
7.6.2 The effect of a shortage or excess of the primary nutrients (N, P and K) on plants
| N (nitrogen) | P (phosphorus) | K (potassium) | |
| Shortage |
|
|
|
| Excess |
|
|
|
7.7 Alternatives to inorganic fertilisers
Although fertilisers are essential for the fast growth of high quality crops, the negative effects of inorganic compounds on the environment must be taken into account. Alternative sources of organic nutrients that can be used to ensure good crops are:
- Bone meal;
- Animal manure;
- Natural plant compost;
- Bat guano (faeces);
- Fish emulsions;
- Kelp meal
Advantages of organic fertilisers:
- Break down and release nutrients more slowly than inorganic fertilisers, so there is less chance of the fertiliser leaching into the soil and causing contamination of groundwater;
- Usually cost less and
- Are often available for free.
Disadvantages of organic fertilisers:
- Not enough is available for large scale usage;
- Provide less nutrients – more has to be used;
- Slow release of nutrients sometimes harms plants;
- Slow release may cause nutrients to be available too late in the plant’s growth cycle.
Activity 3
Fertilisers allow farmers to grow crops in the same soil year after year. However, environmental problems, such as eutrophication, are associated with the application of fertilisers.
- State ONE PRECAUTION that a maize farmer can take to prevent eutrophication. (1)
Nitric acid is an important reactant in the production of ammonium nitrate, a nitrogen-based fertiliser. - Write down the name of the industrial process for the production of nitric acid. (1)
- Write down a balanced equation for the preparation of ammonium nitrate from nitric acid. (3) [5]
Solutions
- Use fertilisers sparingly / Do not over-fertilise
Make use of precision (computerised) application of fertilisers
Ensure that water from fields does not run into rivers/dams
Redirect water from fields into reservoirs/away from rivers/dams (any one) (1) - Ostwald process (1)
- HNO3 + NH3 → NH4NO3 (3) [5]
Activity 4
The rapidly increasing human population is resulting in an ever-increasing demand for food. To meet this demand, farmers apply fertiliser to the same cultivated land EACH YEAR.
- Explain why farmers have to apply fertilisers to their land each year. (1)
- Write down one negative impact that over-fertilisation can have on humans. (1)
- Sulphuric acid is an important substance used in the manufacture of fertilisers.
The equation below represents one of the steps in the industrial preparation of sulphuric acid.
2SO2(g) + O2(g) ⇋ 2SO3(g) ∆H<0
3.1 Write down the name of the process used to prepare sulphuric acid in industry (1)
3.2 Write down the name or the formula if the catalyst used in 2.3.1 (1)
3.3 Is the forward reaction endothermic or exothermic? Give a reason for your answer. (1)
3.4 Write down the name or formula of the fertiliser formed when sulphuric acid reacts with ammonia. (2) [7]
Solutions
- Fertilisers replenish nutrients depleted by the growing of crops (1)
- Damage to crops/soil resulting in small or no harvest/ less income. Excessive fertiliser seeps into groundwater and contaminates drinking water or runs into rivers and/or dams and causes eutrophication which may result in less income /famine/starvation /poor quality drinking water /fewer recreation areas/ environmental damage/ death of wild animals (any one) (1)
- 3.1 Contact process (1)
3.2 V2O5 vanadium pentoxide (any one) (1)
3.3 Exothermic as∆H < 0 (1)
3.4 (NH4)2SO4 OR / Ammonium sulphate (2) [7]
Activity 5
Ammonia, ammonium nitrate and ammonium sulphate are three important nitrogen-containing fertilisers. The flow diagram below shows how these fertilisers are produced in industry.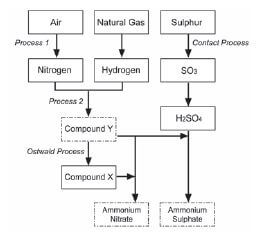
- Use the information in the flow diagram above and write down the following:
1.1 Name Process 1 (1)
1.2 Balanced equation for Process 2 (3)
1.3 Name or Formula for compound X (1)
1.4 Balanced equation for the preparation of ammonium sulphate using sulphuric acid and compound Y (3)
1.5 Name or Symbol of the primary nutrient in ammonium sulphate (1) - Write down one positive impact of fertilisers on humans (1)
- Write down two negative impacts of the use of ammonium nitrate as fertiliser, on humans. (2) [12]
Solutions
- 1.1 Fractional distillation of liquid air (1)
1.2 N2 + 3H2 → 2NH3 (reactants products balance (3)
1.3 Nitric acid / HNO3 (1)
1.4 H2SO4 + 2NH3 → (NH4)2SO4 (reactants products balance (3)
1.5 Nitrogen / N (1) - Enhance growth of crops/plants to produce more food for humans food security for humans production / application of fertiliser results in job creation selling of fertilisers stimulates the economy (any one) (1)
- (Excessive) nitrates in water (eutrophication) can result in blue baby syndrome or cancer (Excessive) nitrates/ammonium ions in water can result in poor quality drinking water or death of fish or less food or fewer recreational facilities or famine due to killing plants / crops from the excess or excessively changing the pH of the soil and thereby reducing the food production (any two) (2) [12]