ElimuZA Access to Education
THE CHLOR-ALKALI INDUSTRY QUESTIONS AND ANSWERS GRADE 12
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupActivity 1
The electrolysis of saturated sodium chloride can be illustrated as follows:
Hydrogen and chlorine bubble off at the electrodes.
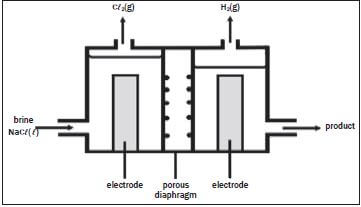
- Give an equation to show how chlorine bubbles are formed at the electrode. (2)
- At which electrode are the chlorine bubbles formed? (1)
- At which electrode does hydrogen gas form? (1)
- What is the name of the product which leaves the cell? (1)
- Give TWO applications of the product formed in 2.4. (2)
- What purpose does the porous diaphragm serve? (2)
- The chlorine gas produced, dissolves in water to form chlorine water. Write down a balanced equation for the reaction that takes place. (2) [11]
Solutions
- 2Cℓ–(aq) → Cℓ2(g) + 2e– (2)
- Anode (1)
- Cathode (1)
- Sodium hydroxide (1)
- Making soap and detergents; paper; rayon and other fibres; dyeing textiles (any 2) (2)
- Stops chlorine passing through; helps to separate sodium hydroxide from NaCℓ(aq). (2)
- Cℓ2 + H2O → HCℓ + HOCℓ (2) [11]
Activity 2
The simplified diagram of a cell used in the chlor-alkali industry is shown below.
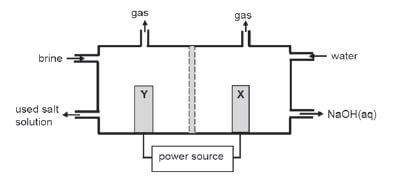
- Write down the CHEMICAL FORMULA of brine. (1)
- At which electrode, X or Y is chlorine gas formed? (1)
- Write down a half-reaction that explains the formation of hydrogen gas at one of the electrodes. (4)
- The purity of the sodium hydroxide produced in the chlor-alkali industry depends on the extent to which it is separated from the chlorine gas produced by this cell. Briefly describe how chlorine gas and sodium hydroxide are prevented from mixing in this cell. (2)
- Apart from the advantages and disadvantages of products produced, write down for this process:
5.1 ONE positive impact on humans. (1)
5.2 ONE negative impact on humans (1) [10]
Solutions
- NaCℓ (aq) (1)
- Y (1)
- 2H2O + 2e– →H2+2OH – (4)
- The membrane prevents chloride ions from moving to the cathode, only allows positive ions through. (2)
- 5.1 Job creation resulting in more people having a better life. (1)
5.2 Uses huge amounts of electricity resulting in load shedding.
OR
Chemical plant uses a lot of space that could have been used for housing/gardens, etc. (any one) (1)