ACIDS AND BASES QUESTIONS AND ANSWERS GRADE 12
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupActivity 1
1. An Arrhenius acid is a substance that
- Accept a proton
- Donates a proton
- Produces H+ in an aqueous solution
- Produces OH– in an aqueous solution (2)
2. Which of the following is an example of the strong base
- CaCO3
- KOH
- K2CO
- NaHCO3 (2)
An aqueous solution that contains more hydronium ions than hydroxyl ions is a(an)
- Acidic solution
- Neutral solution
- Basic solution
- Standardised solution (2)
A solution that has a large amount of dissolved substances in proportion to the volume of water
- Strong solution
- Weak solution
- Concentrated solution
- Diluted solution (2) [8]
Solutions
- D✓✓
- B ✓✓
- A✓✓
- C✓✓ [8]
Activity 2
1. Which of the following is the property of an acid
- Decreases H3O+ ion concentration in solution
- Decreases OH– ion concentration in solution
- Increases OH– ion concentration in solution
- Increases the pH of a solution (2) [2]
Solution
1. B✓✓ [2]
Activity 3
1. In the reaction: H2SO4(aq) + H2O(ℓ) ⇌ HSO4–(aq) + H3O+(aq), the Brønsted-Lowry bases are:
- H2O and H3O+
- H2SO4 and H3O+
- HSO4– and H3O+
- H2O and HSO4– (2) [2]
Solution
1. D ✓✓ [2]
Activity 4
Find the conjugate bases and conjugate acids.
| Acid | Conjugate base | Base | Conjugate acid |
HCℓ | Cℓ− | ||
HNO3 | NO3− | ||
H2SO4 | HSO4− | ||
HSO4− | SO42− | ||
H3PO4 | H2PO4− | ||
H2PO4− | HPO42− | ||
HPO42− | PO43− | ||
H2CO3 | HCO3− | ||
HCO3− | CO32− | ||
CH3COOH | SO42− | ||
(COOH)2 | HSO4− | ||
H2O | OH− | ||
NH4+ | NH3 | ||
H3O+ | H2O |
[28]
Note that some of these are marked in BOLD. That is because they are “amphiprotic”. We will see what this means later on.
Solution
1. Check your answers
Acid | Conjugate base |
HCℓ | Cℓ− |
HNO3 | NO3− |
H2SO4 | HSO4− |
HSO4− | SO42− |
H3PO4 | H2PO4− |
H2PO4− | HPO42– |
HPO42− | PO43− |
H2CO3 | HCO3− |
HCO3− | CO32− |
CH3COOH | CH3COO− |
(COOH)2 | C2O4H− |
H2O | OH− |
NH4+ | NH3 |
H3O+ | H2O |
Base | Conjugate acid |
Cℓ − | HCℓ |
NO3− | HNO3 |
HSO4− | H2SO4 |
SO42− | HSO4− |
H2PO4− | H3PO4 |
HPO42− | H2PO4− |
PO43− | HPO42− |
HCO3− | H2CO3 |
CO32− | HCO3− |
SO42− | HSO− |
HSO4− | H2SO4 |
OH− | H2O |
NH3 | NH4+ |
H2O | H3O+ |
[28]
Activity 5
In the acid-base equilibrium formed by adding HSO4– and OH– the acids are:
- HSO4– and H2SO4
- HSO4– and H2O
- SO42– and H2SO–
- SO42– and H2O (2)
Which of the following is amphiprotic in water?
- SO2
- SO32–
- HSO3–
- H2SO3 (2) [4]
Solutions
1. B✓✓ 2. C✓✓ [4]
Activity 6
Do you think a strong acid will have larger or smaller Ka value? Explain your answer. (3) [3]
Solution
- The strong acid will have a larger Ka value.
- A strong acid is a better proton donor, resulting in more products.
- Since the concentration of the products is in the numerator of the Ka expression, the stronger the acid, the larger the Ka. [3]
Activity 7
Choose the strongest base in the list below by comparing their Kb values.
Base Kb
- Ammonia, NH3 1,8 × 10–5
- Hydroxylamine, HONH2 9,1 × 10–9
- Ethylamine, C2H5NH2 4,3 × 10–4 (2) [2]
Solution
- C [The larger the Kb, the stronger the base] [2]
Solids and pure liquids are NEVER written in the equations for the equilibrium constant Kc, or Ka or Kb as their concentration is [1]
Activity 8
- A standard solution is a solution ...
- at 25˚C
- of an acid or a base
- of which the volume is known
- of which the concentration is known (2)
- Consider the following ionisation equilibrium:
H2O(ℓ) ⇋ H+(aq) + OH−(aq)
The ionisation constant of water (Kw) in the above reaction increases from 1 × 10–14 at 25˚C to 9,6 × 10–14 at 60˚C. Which one of the following statements is therefore correct- [H+] > [OH−] at 60˚C
- The ionisation reaction is exothermic
- The pH increases with an increase in temperature
- The pH decreases with an increase in temperature (2)
- Consider the reaction:
CH3COOH(g) + H2O(ℓ) ⇋ H3O+(aq) + CH3COO–(aq)
The ionisation constant for this reaction at 25°C is 1,8 × 10–5. The equilibrium constant for this reaction at 60°C, is 3,6 × 10–7. From this information we can deduce that forward reaction ...
(Explain your choice).- is endothermic
- is exothermic
- is a redox reaction
- is a precipitation reaction (2)
- Two beakers, A and B, contain solutions of the same concentration with a pH of 2 and 4,5 respectively. Which of the following combinations is correct?
Beaker A Beaker B- Weak Acid Strong Acid
- Strong Acid Weak Acid
- Strong base Weak base
- Weak base Strong base (2)
- Andile rinses the apparatus before starting an acid-base titration experiment. Which rinsing method is likely to cause inaccurate results?
- The Erlenmeyer (conical) flask is rinsed with distilled water
- The burette is rinsed with the acids it is to be filled with
- The pipette is rinsed with the base it is to be used for
- The volumetric (measuring) flask, which is used to make up the standard solution of the base, is rinsed with distilled water. (2)
- If base X is titrated against acid Y, the pH of the solution at the end point is 8. The base X and acid Y are respectively:
X Y- NaOH CH3COOH
- Na2CO3 HCℓ
- NaOH H2SO4
- Na2CO3 CH3COOH (2) [12]
Solutions
- D ✓✓(2)
- D
 (2)
(2) - B✓✓ Kc at 25°C is greater than Kc at 60°C
Kc = [CH3COO–][ H3O+]. If the mixture is heated, the Kc decreases (2) - B✓✓ Beaker A has a low pH which indicates that it contains more H+, therefore it has ionised completely. Whilst beaker B has a high pH which indicates few H+ ions, thus it has partially ionised. (2)
- A✓✓ The Erlenmeyer (conical) flask must be rinsed with distilled water. Only the number of moles of base which was measured in the pipette must be neutralised. (2)
- A✓✓ (2) [12]
Activity 9
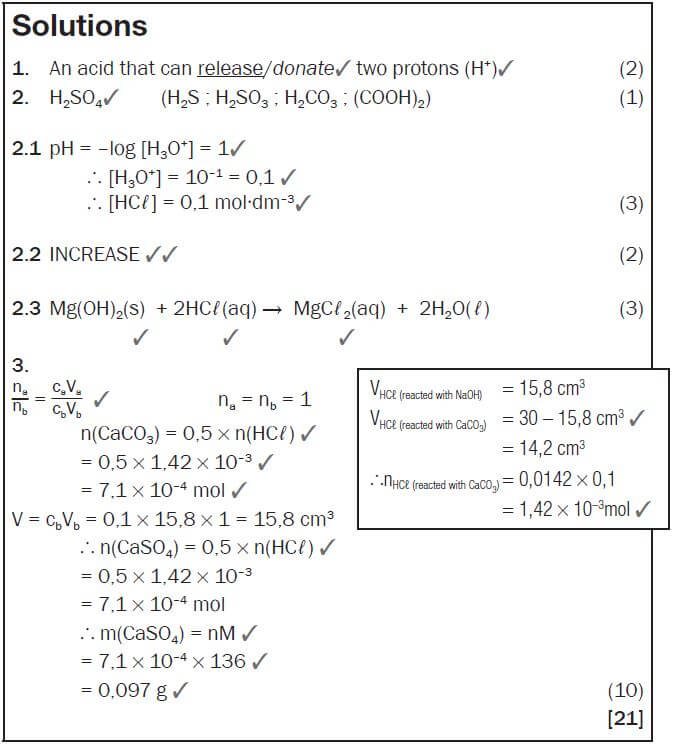
- Write down:
1.1 The meaning of the term diprotic acid. (2)
1.2 The formula of a diprotic acid. (1) - Magnesium hydroxide (Mg(OH)2) is often used as medicine to relieve an upset stomach. The pH of the HCℓ(aq) in a person’s stomach is 1.
2.1 Calculate the concentration of the hydrochloric acid in the person’s stomach. (3)
2.2 Will the pH in the stomach INCREASE, DECREASE or STAY THE SAME after taking in a dose of Mg(OH)2? (2)
2.3 A person takes in a dose of Mg(OH)2. Write down the balanced equation for the reaction that takes place in the stomach. (3) - A textbook states that calcium sulphate (CaSO4) is slightly soluble in water. Two learners decided to test the dam water from a local municipality for calcium sulphate. They took a 0,5 dm3 sample of the dam water and treated it with sodium carbonate solution to precipitate the calcium ions present according to the following equation:
CaSO4(aq) + Na2CO3(aq) → Na2SO4(aq) + CaCO3(s)
The precipitate is then dissolved in 30 cm3 of 0,1 mol·dm–3 HCℓ solution which converts the precipitate to aqueous calcium chloride, water and carbon dioxide according to the following equation:
CaCO3 + 2HCℓ → CaCℓ2 + CO2 + H2O
The HCℓ was in excess. They neutralised the excess HCℓ by adding 15,8 cm3 of a 0,1 mol·dm–3 NaOH solution. The equation for the reaction is:
HCℓ + NaOH → NaCℓ + H2O
Calculate the mass of calcium sulphate that was present in the sample of dam water. (10) [21]