ORGANIC COMPOUNDS AND MACROMOLECULES QUESTIONS AND ANSWERS GRADE 12
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupActivity 1
For each of the following isomers:
Write down the structural formula
Identify the type of isomer
1. 2-methylpentane and 3-methylpentane.
2. 1-chlorobutane and 1-chloro-2-methylpropane
3. Propanoic acid and ethyl methanoate (13) [13]
Solutions:
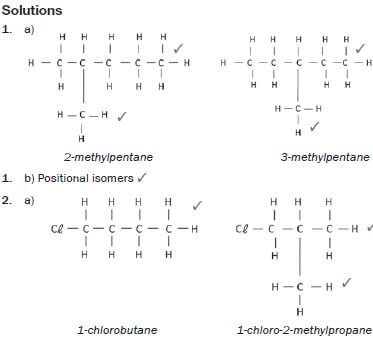
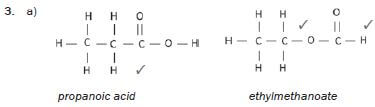
hint
Structural isomers have different IUPAC names and different physical properties.
Activity 2
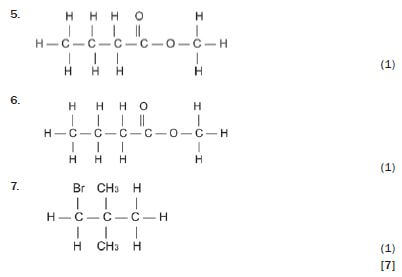
Write down the IUPAC names for the following organic molecules.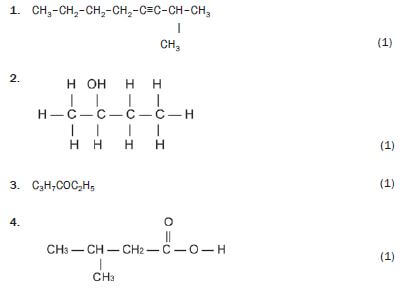
Solutions
1. 2-methyloct-3-yne 3 (1)
2. butan-2-ol 3 (1)
3. butanal 3 (1)
4. hexan-3-one 3 (1)
5. 3-methyl-butanoic acid 3 (1)
6. methyl butanoate 3 (1)
7. 1-bromo-2,2-dimethyl-propane 3 (1) [7]
Important to remember:
- When naming haloalkanes, the halogen atoms do not get preferenceover alkyl groups – numbering should start from the side nearestto the first substituent, either the alkyl group or the halogen. Inhaloalkanes, where e.g. a Br and a Cℓ have the same number whennumbered from different sides of the chain, Br gets alphabeticalpreference.
- When writing IUPAC names, substituents appear as prefixes writtenalphabetically (bromo, chloro, ethyl, methyl), but the prefixes di- andtri- should not be used to determine the alphabetical order.
- In molecules where the functional group is ALWAYS on the firstcarbon (such as in the case of carboxylic acids, aldehydes there isNO number added to indicate the position of the fuctional group.(e.g. Ethanoic acid or pentanal.)
Activity 3
1. Which ONE of the following formulae represents an alkane?
- C2H2
- C3H4
- C3H6
- C3H8 (2)
2. An organic compound has the structural formula shown below: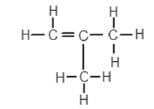
The correct systematic (IUPAC) name for the compound is …
- but-1-ene.
- but-2-ene.
- methylpropene.
- methylpropane.
3. Which ONE of the following compounds can exist as an isomer?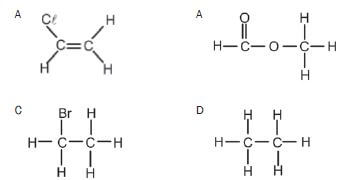 (2)
(2)
An example of an unsaturated hydrocarbon is:
- C2HCℓ3
- C3H6
- C2H6
- C2H5OH (2) [8]
Solutions
- D ✓✓ (2)
- C ✓✓ (2)
- B ✓✓ (2)
- B ✓✓ (2) [8]
Activity 4
Consider the following list of organic compounds: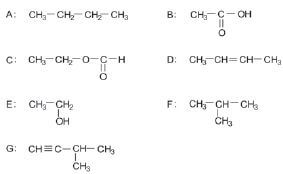
1. Using structural formulae, write an equation for the preparation of an ester. Choose the reactants from the above list. (5)
2. Write the letters representing TWO compounds in this list that are isomers. (2)
2.1 C
2.2 D (2)
3. Write down the letter that represents the compound that is formed when ethanol is oxided. (1)
4. Give one use of esters. (1) [11]
Solutions
1. 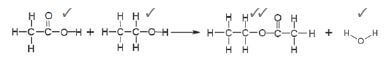
2. A ✓ & F ✓ (5)
2.1 ethylmethanoate ✓ (2)
2.2 but-2-ene ✓ (2)
3. B ✓ (1)
4. Manufacturing of perfumes/flavourants ✓ (1) [11]
Activity 5
1.1 The boiling points of branched alkanes are lower than those of straight chain alkanes containing the same number of carbon atoms because branched alkane chains have
- Large molecular masses
- Longer chain lengths
- More electrons
- Smaller effective molecular surface areas (2)
1.2 Which ONE of these compounds has the highest vapour pressure at room temperature?
- Propane
- Ethane
- Ethanol
- Fluoroethane (2)
2. Will the boiling point of hexane be HIGHER THAN or LOWER THAN that of pentane? Refer to MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and ENERGY needed to explain the answer. (4)
3. How will the boiling point of an ISOMER of Butane compare to that of Butane? Write down HIGHER THAN, LOWER THAN or EQUAL TO. Refer to MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and the ENERGY needed to explain the answer. (4)
4. Consider the boiling points of compounds propan-1-ol (97˚C) and ethanoic acid (118˚C).
4.1 Give a reason for this difference in boiling points by referring to the intermolecular forces present in EACH of these compounds. (3)
4.2 Which ONE of the compounds propan-1-ol or ethanoic acid has a higher vapour pressure? Refer to their boiling points to give a reason for the answer. (3) [18]
Solutions
1.1 D ✓✓(2)
1.2 A ✓✓ (2)
- Higher ✓
Structure: Hexane has a longer chain length than pentane. OR Hexane contain more C- atoms than pentane. OR Hexane has a greater molecular size than pentane. OR Hexane has a larger surface area than pentane. ✓
Intermolecular forces: Between the different molecules of hexane you will find stronger or more intermolecular forces than between the different molecules of pentane. ✓
Energy: More energy needed to overcome or break intermolecular forces between hexane molecules than you need between pentane molecules. ✓ (4)
3. Lower than ✓
- Isomers of Butane: More branching / Smaller surface area (over which the intermolecular forces act.) ✓ Weaker/less intermolecular forces. ✓ Less energy needed to overcome intermolecular forces. ✓ (4)
4.
4.1 Ethanoic acid: Two sites for hydrogen bonding/forms dimers ✓ Propan-1-ol: One site for hydrogen bonding. ✓ Therefore it will require more energy to break the bonds between the ethanoic acid molecules than to break the bonds between
the propan-1-ol molecules, ✓ which explains why ethanoic acid has the lower boiling point. (3)
4.2 Propan-1-ol has the highest vapour pressure. ✓Because propan-1-ol has a lower boiling point it means less energy is required to break the bond between the particles of propan-1-ol ✓and therefore more molecules will be present in the vapour state and therefore vapour pressure will increase. ✓ (3) [18]
Activity 6
1. Unsaturated vegetable oils are hardened to make margarine. The reaction that takes place during the hardening process can be described as:
- Addition
- Hydrogenation
- Substitution
- I and II
- II and III
- Only I
- Only II (2)
2. Which ONE of the following compounds has the highest melting point?
- Butane
- Butene
- 1-bromobutane
- 1-methylpropane (2)
3. Which ONE of the following pairs of compounds correctly represents the products formed during the COMPLETE combustion of Octane?
- CO and H2O
- CO and H2
- CO2 and H2
- CO2 and H2O (2)
4. Most organic compounds can undergo substitution or addition or elimination reactions to produce a variety of organic compounds. Some incomplete reactions are shown below: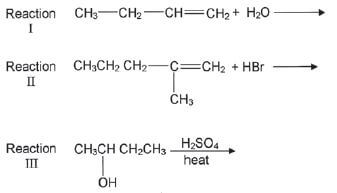
4.1 Name the type of reaction represented by Reaction III. (1)
4.2 Both Reactions I and II are examples of addition reactions. Name the type of addition reaction that is represented by each reaction. (2)
4.3 Write down the IUPAC name of the major product formed in Reaction I. (1)
4.4 Reaction I only takes place in the presence of a catalyst. Write down the formula of the catalyst used in Reaction I. (1)
4.5 Write down the structural formula and the IUPAC name of the major product in Reaction II. (3)
4.6 To which homologous series does the organic product formed in Reaction III belong? (1) [15]
Solutions
1. A ✓✓ (2)
2. C ✓✓ (2)
3. D ✓✓ (2)
4.1 Elimination ✓ (1)
4.2 I- hydration ✓ II- hydrohalogention 3 (2)
4.3 Butan-2-ol ✓ (1)
4.4 H2SO4 ✓ (1)
4.5 2-bromo-2-methylpentane ✓ (3)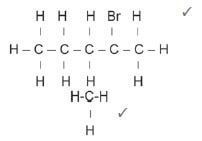
4.6 Alkene ✓ (1) [15]
Activity 7
1. Give the definition of a macromolecule (1)
2. Explain the difference between a monomer and a polymer. (2)
3. What is the chemical reaction in which a monomer molecules join to form a polymer called? (1)
4. The polymer below is the product of a polymerisation reaction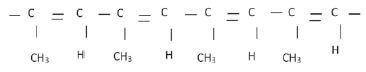
4.1 What is the IUPAC name of the monomer used to form this polymer? (1)
4.2 Give the structural formula of the monomer used to form this polymer. (2)
4.3 Is this an example of an addition or condensation polymerisation? Give a reason for your answer. (2)
5. A carboxylic acid monomer and an amine monomer has joined in an amide linkage as shown below.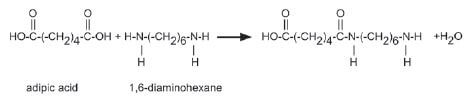
Name the type of polymerisation that occurs in this equation. Give a reason for your answer. (2)
6. Write an equation for polymerisation of ethane to form polyethene. (2)
7. Is (6) an example of addition or condensation polymerisation? Give a reason for your answer. (2)
8. Name at least three industrial uses of polythene. (3) [18]
Solutions
1. A molecule that consists of a large number of atoms. ✓ (1)
2. A polymer is a large molecule that is made up of smaller units, covalently bonded to each other in a repeating pattern. ✓Each of these smaller units are called a momomere. ✓ (2)
3. Polymerisation ✓ (1)
4. 4.1 prop-(1)-yne (1)
4.2 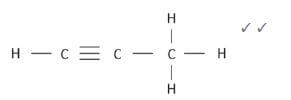 (2)
(2)
4.3. Addition polymerisation 3 monomers combined through an addition reaction. ✓ (2)
5. Condensation polymerisation. Two monomers with different functional groups are joined ✓ and when they join it leads to the “loss” of a small molecule, in this case, water. ✓ (2)
6. 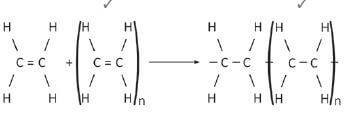 (2)
(2)
7. Addition polymerisation ✓ monomers combinded through an addition reaction. ✓ (2)
8. Any of the following: sandwich bags, ✓cling wrap, ✓ car covers, ✓ squeeze bottles, liners for tanks and ponds, moisture barriers in construction, freezer bags, water pipes, wire and cable insulation, extrusion coating. (3) [18]