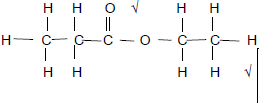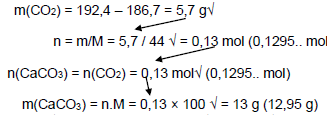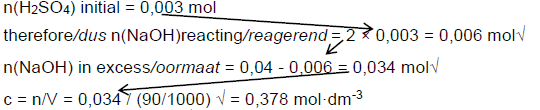PHYSICAL SCIENCES CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS JUNE 2016
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES P2
GRADE
JUNE2016
MEMORANDUM
NSC Past Papers And Memos
QUESTION 1
1.1 C√√ 1.2 A √√ 1.3 B√√ 1.4 D√√ 1.5 C√√
1.6 A√√ 1.7 A√√ 1.8 A√√ 1.9 B√√ 1.10 D√√ [20]
QUESTION
2.1.1 CnH2n√ (1)
2.1.2 Ketone√(1)
2.1.3 E√ (Methanal) (1)
2.2.1 Contains double bonds (or multiple bonds) between C atoms. √√ (2)
2.2.2 Addition√(1)
2.2.3 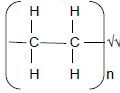
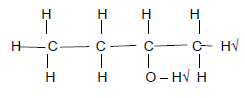
2.3.1 2-bromo√-4-methyl√ hexane√(3)
| Marking criteria 2-bromo√ or 2 bromo 4-methyl√ or 4 methyl hexane√ Any error e.g. omission of hyphens, incorrect order etc. (2/3) |
2.3.2 4-methyl√pentan-2-one√ Accept 4-methyl-2-pentanone (2)
| Marking criteria 4-methyl√ or 4 methyl pentan-2-one√ or pentan 2 one n Any error e.g. omission of hyphens, incorrect order etc. (1/2) |
2.4.1
| Marking criteria Functional group√ Whole structure correct√ |
2.4.2 2-methyl√propan-2-ol√ Accept 2-methyl-2-propanol (2)
| Marking criteria 2-methyl√ propan-2-ol√ |
2.5.1 Propanoic acid√(1)
2.5.2
| Marking criteria Functional group√ Whole structure correct√ |
2.6.1 C2H4Br2√ (1)
| NOTES: Ignore order of atoms in formula C2Br2H4 √ |
2.6.2
| Positive marking from 2.6.1 |
% C = (2x12)/188 × 100 √
= 12,76% or/of 12,77% √
% H = (4×1)/188 × 100√
= 2,13% or/of 2,12%√
% Br = 100 – (12,76 + 2,13) = 85,11%√
OR
% Br = (2×80) /188 × 100
= 85,11% or/of 85,10%√ (6)
[27]
QUESTION 3
3.1 Positional√ (isomer) (1)
3.2.1 Substitution√ (1)
3.2.2 Hydrogen bromide√/HBr(1)
3.3.1 (concentrated) potassium hydroxide√/KOH(1)
3.3.2 strong heat√(1)
3.4 Hydration√(1)
3.5 (concentrated) H2SO4√/Sulphuric acid (1)
3.6 C4H8 + 6O2 √ → 4 CO2 + 4H2O√ √ Balancing(3)
[10]
QUESTION 4
4.1 A√ (Butane) (1)
4.2 A group of organic compounds with the same functional group√√ / where one member differs from the next member with a –CH2– group.(2)
| NOTES 2 marks or 0 |
4.3 London force√ /induced-dipole force/dispersion force (1)
4.4.1 In A there are London forces√
In B there are dipole-dipole forces √ (in addition to London forces)
Intermolecular forces in B are stronger√ than in A.OR Intermolecular forces in A are weaker than in B. (3)
4.4.2 Both C and D have hydrogen bonds√
Hydrogen bonds are stronger in D than C√ because D has more sites for
Hydrogen bonds√ /two sites for hydrogen bonding/ D forms dimers/D is more polar. (3)
4.5.1 D√ (Butanoic acid) (1)
4.5.2 Compound E has a larger surface area than D√/longer carbon chain length than D.
London forces√/induced dipole/dispersion forces in E are stronger√ than in D.
OR
London forces/induced dipole/dispersion forces in D are weaker than in E. (3)
[14]
QUESTION 5
5.1 HETEROGENEOUS√(1)
5.2 CO2 escapes√(1)
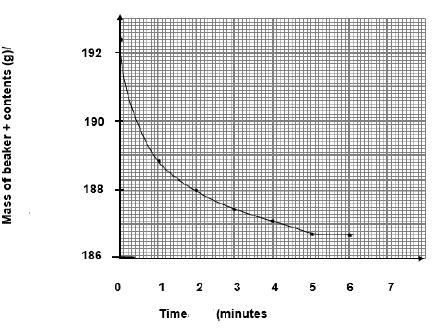
5.3 t = 5√ (minutes) (1)
5.4 Rate= - Δm/Δt = -(188,8–192,4) √ /(1-0) √
= 3,6 √ (grams per minute).
Accept
Rate = Δm/Δt = (188,8–192,4) √ /(1-0) √
= -3,6 √ (grams per minute) (3)
5.5 Concentration of the acid decreases√/Surface area of CaCO3 decreases.√ (2)
5.6 Calcium carbonate√/CaCO3
5.7
Accept Range 12,95 g to g
5.8 Graph of mass of beaker and contents vs time(4)
5.9 Reaction rate increases√
Average kinetic energy of particles increases √ (as temperature increases)
More particles have enough kinetic energy to react√
More effective collisions per unit time√(4)
[22]
QUESTION 6
6.1 Stage reached by a reversible chemical reaction in a closed container where the rate of forward reaction is equal to rate of reverse reaction√√ (2)
| NOTES: 2 marks or 0 |
6.2.1 4 √ (minutes) (1)
6.2.2 0,5 √ (mol) (1)
6.3
Marking criteria
- division all n by V√.
- Kc expression√/
- substitution for Kc√
- substitution of equilibrium concentrations√√.
- final answer√
Concentration at equilibrium
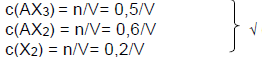
Kc = [AX2]2.[X2]/[AX3]2 √
2,5 × 10-2 = (0.6/V)2. (0,2/V)√ / (0.5/V)2 √
V= 11,52 dm3√
(6)
6.4 Low√ Kc is less than 1 (or Kc is low) (2)
6.5 Endothermic√
As temperature decreases [AX3] increases√
Reverse reaction is favoured by decrease in temperature√
Reverse reaction is exothermic√ (4)
6.6 Remains constant√. (1)
[17]
QUESTION7
7.1.1 Ionisation constant√ (for an acid)(1)
7.1.2 Ka depends on temperature√/Ka changes as temperature changes.(1)
7.1.3 H(COO)2- √ (1)
7.1.4 (COOH)2(s) + 2H2O(ℓ) √ ⇌ (COO)2 2-(aq) + 2H3O+(aq) √
√ Balancing(3)
| Notes Ignore phases Ignore type of arrow |
7.2.1
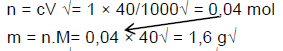
7.2.2 n = c.V√ = 0,06 × 50/1000√ = 0,003 mol√ (3)
7.2.3Positive marking from 7.2.1 and 7.2.2
Marking criteria
- use of ratio n(H2SO4):n(NaOH)√
- n(NaOH) in excess n initial – n reacting√
- substituting (90/100) into c = n/V√
- [OH-].[H3O+] = 10-14√
- substitution for/vervanging vir [OH-]√
- pH = -log[H3O+]√
- substitution for [H3O+]√
- final answer √
n(NaOH) initial = 0,04 mol therefore n(H2SO4) required = ½ × 0,04 = 0,02 mol
[OH-].[H3O+] = 10-14√
0,378√ × [H3O+] = 10-14
[H3O+] = 2,65 × 10-14
pH = -log[H3O+]√
= -log 2,65 × 10-14√
= 13,58√ (8)
7.3.1 Endpoint√(1)
7.3.2 Burette√(1)
7.3.3 To see endpoint clearly.√ (1)
7.3.4 CH3COO- + H2O √ ⇌ CH3COOH + OH-√ √ balancing
Ethanoate ion reacts with water to form (an excess of) OH-ions√ (4)
[28]
QUESTION 8
8.1 Activation√ (energy) (1)
8.2.1 Exothermic√ ΔH < 0 √ / Δ H negative (2)
8.2.2 Increase pressure.√ / Decrease temperature√ / Add more NO or O2 √ (any 2) (2)
8.2.3 ΔH = -74,55 √ (1)
8.3.1 Neutralisation√ /Protolysis/Protolytic (1)
8.3.2 Proton √ (1)
8.3.3 n(NaOH) = n(NH4NO3) = 2,4 × 10-3 mol√
m(NH4NO3) = n.M = 2,4 × 10-3 × 80√ = 0,192 g
% Purity = 0,192 / 0,204 × 100√ = 94,12 %√ (4)
[12]
TOTAL : 150