PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC EXAMS PAST PAPERS AND MEMOS JUNE 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES
PAPER 2
GRADE 12
NSC EXAMS
PAST PAPERS AND MEMOS JUNE 2019
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of SEVEN questions.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub-questions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions. Each question has only ONE correct answer. Write only the letter (A–D), corresponding to the correct answer of your choice, next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 Which ONE of the following compounds has a DOUBLE BOND in its structure?
- Ethane
- Ethene
- Polyethene
- Bromoethane (2)
1.2 By definition the boiling point of a liquid is the temperature at which …
- a liquid changes to vapour.
- the vapour pressure equals atmospheric pressure.
- the vapour pressure is less than the atmospheric pressure.
- the vapour pressure is greater than the atmospheric pressure. (2)
1.3 Which ONE of the following is the CORRECT IUPAC name of the compound shown below? 
- 2-bromobutane
- 2-methyl-2-bromopropane
- 2-bromo-2-methylpropane
- 2,2,2-trimethylbromomethane (2)
1.4 Consider the reaction represented by the equation below:
CH3Br + H2O → CH3OH + HBr
The TYPE of reaction shown above is …
- substitution.
- combustion.
- esterification.
- polymerisation. (2)
1.5 The boiling points of propanal and propanone are compared.
Which ONE of the following is the INDEPENDENT variable in this comparison?
- Molar mass
- Chain length
- Hydrogen bonds
- Homologous series (2)
1.6 Which ONE of the following factors will change the value of the equilibrium constant, Kc?
- Pressure
- Surface area
- Temperature
- Concentration (2)
1.7 The reaction of magnesium powder and hydrochloric acid is used to investigate the factors that affect reaction rate. The balanced equation for the reaction is:
Mg(s) + 2HCℓ (aq) → MgCℓ2(aq) + H2(g)
In ALL the experiments the hydrochloric acid is in EXCESS and the magnesium powder is completely covered.
The results of the experiments are shown in the table below:
Experiment | Concentration of HCℓ | Volume of | Mass of |
P | 1,5 | 200 | 1,8 |
Q | 1,2 | 400 | 1,8 |
R | 0,8 | 200 | 1,4 |
S | 1,5 | 200 | 1,5 |
Which experiments will produce the SAME amount of hydrogen at same temperature and pressure?
- P and Q
- Q and R
- R and S
- P and S (2)
1.8 Diluted hydrochloric acid is spilt on floor tiles in the laboratory. Which ONE of the following substances can be used to neutralise the acid?
- NaCℓ
- NH4Cℓ
- H2SO4
- Na2CO3 (2)
1.9 Study the ionisation reactions given below:
H3PO4 + H2O → H2PO4- + H3O+
H2PO4- + H2O → HPO42- + H3O+
In the reactions H2O acts as a(n) …
- base.
- ampholyte.
- weak acid.
- strong acid. (2)
1.10 The potential energy diagram shown below is for the reversible hypothetical reaction shown below:
2 AB(g) ⇌ A2B2(g) 
Consider the following statements about the reaction:
- The forward reaction is endothermic
- The value of ΔH for the REVERSE reaction is equal to q
- The activation energy for the reverse reaction is equal to p + q Which of the above statements is/are TRUE?
- I only
- I and II
- III only
- II and III (2) [20]
QUESTION 2
A test tube containing an alcohol P, carboxylic acid R and sulphuric acid is heated in a water bath, as illustrated below. A piece of wet cotton wool is placed at the mouth of the test tube.
Alcohol P + Carboxylic acid R Ester + X 
2.1 Is the sulphuric acid that is used CONCENTRATED or DILUTE? (1)
2.2 What is the purpose of placing cotton wool at the mouth of the test tube? (1)
2.3 Write down the molecular formula of the inorganic product, X. (1)
2.4 Alcohol P is a positional isomer of propan-2-ol. Write down the IUPAC name of alcohol P. (2)
2.5 Chemical analysis shows that carboxylic acid R contains 54,55% C, 9,1% H and x % O. The molar mass of the ester produced is 130 g·mol-1. Determine by calculation the MOLECULAR formula of carboxylic acid R. (8) [13]
QUESTION 3
3.1 The boiling points of three compounds (A, B and C) are compared in the table below:
COMPOUND | BOILING POINT (°C) | |
A | Pentan-1-ol | 138 |
B | Butanoic acid | 164 |
C | Methyl propanoate | 68 |
3.1.1 For compound C write down the:
- Name of the homologous series to which it belongs (1)
- STRUCTURAL formula (2)
3.1.2 Give a reason why this is a fair comparison. (2)
3.1.3 Explain why hydrogen bonds are stronger in compound B than in compound A. (2)
3.2 The vapour pressure values of a secondary and a tertiary alcohol are compared in the table below:
COMPOUND | MOLECULAR FORMULA | VAPOUR PRESSURE | |
D | Secondary alcohol | C4H10O | X |
E | Tertiary alcohol | C4H10O | Y |
3.2.1 Are compounds D and E STRUCTURAL isomers? Write down Yes or No. Give a reason for the answer. (3)
3.2.2 Which ONE of the two values, X or Y is HIGHER? Fully explain the answer. (4) [14]
QUESTION 4
4.1 Study the two reactions shown below. P is an inorganic compound. R is an organic compound.
- : 3-methylbut-1-ene + P 1,2-dichloro-3-methylbutane
- : 2-bromobutane + Base R + NaBr + H2O
4.1.1 Write down the TYPE of reaction represented by:
- Reaction I (1)
- Reaction II (1)
4.1.2 Write down the:
- STRUCTURAL formula of 1,2-dichloro-3-methylbutane (3)
- Name of the inorganic reactant P (1)
4.1.3 For reaction II write down:
- The structural formula and IUPAC name of the major product, R (4)
- ONE reaction condition (1)
4.2 The equation shown below shows n molecules of H2C=CH2 reacting to produce a polymer. H2C=CH2 in the equation below represents small organic molecules that can be covalently bonded to each other in a repeating pattern
Polymerisation
n H2C=CH2 → [CH2CH2] 1000
4.2.1 Is this reaction an example of ADDITION or CONDENSATION polymerisation? (1)
Write down:
4.2.2 The value of n (1)
4.2.3 A term used for the underlined phrase (1)
4.2.4 ONE use of the polymer produced in this reaction (1)
4.3 The following equation represents the cracking reaction of octane (C8H18). Y is a straight chain organic compound.
High temperature/High pressure
C8H18 → Y + C2H4
4.3.1 Define the term cracking reaction. (2)
4.3.2 Is this reaction an example of THERMAL CRACKING or CATALYTIC CRACKING? (1)
4.3.3 Write down the STRUCTURAL FORMULA and IUPAC name of compound Y. (4) [22]
QUESTION 5
Hydrogen peroxide (H2O2) decomposes according to the following equation:
2H2O2(aq) 2H2O(ℓ) + O2(g)
5.1 State THREE factors that can INCREASE the rate of this reaction. (3)
5.2 Define the term reaction rate. (2)
The rate of decomposition of hydrogen peroxide is investigated in THREE experiments. The initial temperature of H2O2 is the same in ALL the experiments.
The reaction runs to completion in ALL the experiments.
5.3 In experiment 1 a solution of hydrogen peroxide is heated to 35 °C. The concentration of H2O2 was measured at different time intervals during the experiment. The following results were obtained:
TIME (MINUTES) | H2O2 CONCENTRATION (mol·dm-3) |
0 | 1,9 |
15 | 1,45 |
55 | 1,10 |
100 | 0,85 |
215 | 0,60 |
5.3.1 Calculate the average reaction rate, in mol·dm-3·min-1 during the first 15 minutes. (3)
5.3.2 Give a reason why the average reaction rate is HIGHER during the first 15 minutes, compared to the time interval 100 to 215 seconds. (2)
5.4 In experiment 2, a small amount of manganese dioxide is added to the same H2O2 solution (used in experiment 1) and the mixture is heated to 35 °C. 
5.4.1 Is the reaction EXOTHERMIC or ENDOTHERMIC? (1)
5.4.2 Use the collision theory to explain the effect of manganese dioxide on the rate of decomposition of H2O2. (4)
5.5 In experiment 3 the same volume of the solution of H2O2 (used in experiment 1) is used but ONLY the temperature to which the solution is heated is changed. The Maxwell-Boltzman distribution curves for experiment 1 and 3 are shown below: 
5.5.1 Which experiment (1 or 3) was carried out at a HIGHER temperature? Give a reason for the answer. (2)
5.5.2 How does the area under the graph of experiment 1 compare to the area under the graph of experiment 3? Write down GREATER THAN, EQUAL TO or LESS THAN. Explain the answer. (2)
5.6 In experiment 2 the total volume of oxygen produced at 35 °C is 0,2 dm3. Calculate the mass of H2O2 that decomposed in experiment 2 if the molar volume of oxygen at 35 °C is 24,8 dm3·mol-1. (5)
5.7 The graphs below show how the concentration of H2O2 changes with time in the experiments. 
Which graph (P, Q or R) represents the results of:
5.7.1 Experiment 1 (1)
5.7.2 Experiment 2 (1)
5.7.3 Experiment 3 (1) [27]
QUESTION 6
Carbon dioxide gas, CO2(g), reacts with carbon, C(s), according to the following balanced equation:
CO2(g) + C(s) ⇌ 2CO(g)
The system reaches equilibrium at 1 000 °C.
6.1 Define the term chemical equilibrium. (2)
6.2 How will EACH of the following changes affect the number of moles of CO(g) at equilibrium? Choose from INCREASES, DECREASES or REMAINS CONSTANT.
6.2.1 The addition of more carbon, C (1)
6.2.2 The addition of more CO2 (1)
6.2.3 Decreasing the pressure by increasing the volume (1)
6.3 Use Le Chatelier’s principle to explain the answer to QUESTION 6.2.3 above. (3)
6.4 The sketch graph given below shows the changes in the rates of reaction from the moment the reagents are introduced into the container. The reaction reaches equilibrium for the first time at t1. A change is made to the equilibrium mixture at t2. 
6.4.1 Which reaction (FORWARD or REVERSE) is represented by the broken line? (1)
6.4.2 What change was made at t2? (2)
Initially 104,72 g of CO2 was heated with EXCESS carbon C in a sealed flask of volume 250 cm3. The equilibrium mixture was found to contain 1,90 mol of CO2.
6.5 Calculate the value of the equilibrium constant, Kc at 1 000 °C. (8)
6.6 The value of Kc for the reaction decreases to 0,333 when the temperature is changed to 25 oC.
6.6.1 Is there a HIGH or LOW yield at 25 oC? Give a reason for the answer. (2)
6.6.2 Is the FORWARD reaction ENDOTHERMIC or EXOTHERMIC? Explain the answer fully. (4) [25]
QUESTION 7
7.1 The hydrogen carbonate ion (HCO3-) undergoes hydrolysis according to the equation:
HCO3-(aq) + H2O(ℓ) ⇌ CO32-(aq) + H3O+(aq)
7.1.1 Define the term hydrolysis. (2)
7.1.2 Give a reason why HCO3-is regarded as an ampholyte. (2)
Write down the FORMULA of the:
7.1.3 Conjugate acid of CO32-(1)
7.1.4 Conjugate acid of HCO3-(not in the equation) (1)
7.2 A weak monoprotic acid is dissolved in water to produce a solution of accurately known concentration.
7.2.1 Write down a term for the underlined phrase. (1)
7.2.2 Which ONE of the following is an example of a weak monoprotic acid?
CH3COOH | HCℓ | H2CO3 |
The weak monoprotic acid has a concentration of 0,2 mol.dm-3. (2)
7.2.3 Calculate the volume to which 20 cm3 of the acid must be diluted to produce a solution with a concentration of 0,16 mol·dm-3. (3)
7.3 During a titration a learner adds 30 cm3 of NaOH solution of concentration 0,1 mol.dm-3 to an Erlenmeyer flask and titrates this solution with 25 cm3 HCℓ solution. The balanced equation for the reaction that takes place is:
NaOH + HCℓ NaCℓ + H2O
7.3.1 Name this type of reaction. (1)
7.3.2 At what pH range will a suitable indicator for this titration change colour? Choose from:
6,8 to 7,2 | 3 to 5 | 8,6 to 10 |
Give a reason for the answer. (3)
7.3.3 Calculate the concentration of the HCℓ that was used to neutralise the NaOH in the Erlenmeyer flask. (4)
The learner passes the endpoint by adding an extra 8 cm3 of the HCℓ solution.
7.3.4 Define the term endpoint. (2)
7.3.5 Calculate the pH of the solution in the flask after the addition of the extra 8 cm3 of HCℓ. (7) [29]
TOTAL: 150
NATIONAL SENIOR CERTIFICATE
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,013 × 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
Charge on electron | e | -1,6 × 10-19 C |
Avogadro’s constant | NA | 6,02 × 1023 mol-1 |
TABLE 2: FORMULAE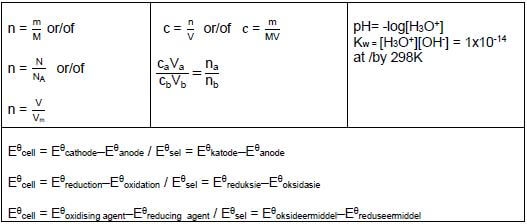
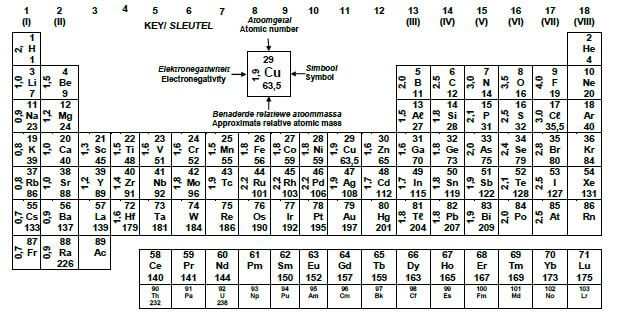
TABLE 3: THE PERIODIC TABLE OF ELEMENTS