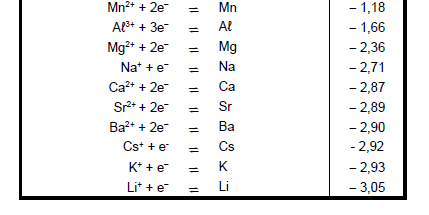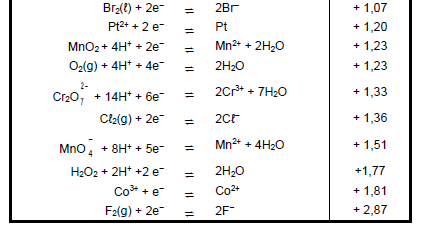PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS NOVEMBER 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY (P2)
GRADE 12
NOVEMBER 2019
NATIONAL SENIOR CERTIFICATE
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 E.
1.1 Which ONE of the following compounds has the HIGHEST vapour pressure?
- HCOOH
- CH3CHO
- CH3CH2OH
- CH3CH2CH3 (2)
1.2 Which ONE of the formulae below represents the product of a POLYMERISATION reaction?
1.3 Which ONE of the following combinations are BOTH UNSATURATED HYDROCARBONS?
- Ethane and ethene
- Ethene and ethyne
- Ethane and ethanol
- Ethanoic acid and ethene (2)
1.4 Which ONE of the following sets of values for activation energy (Ea) and heat of reaction (ΔH) is possible for a reaction?
| ACTIVATION ENERGY (Ea) (kJ∙mol-1) | HEAT OF REACTION (ΔH) (kJ∙mol-1) | |
| A | 100 | +100 |
| B | 50 | +10 |
| C | 50 | +50 |
| D | 100 | -50 |
(2)
1.5 Consider the following balanced equation for a system at equilibrium:
2SO2(g) + O2(g) ⇌ 2SO3(g)
How will the addition of a catalyst to the equilibrium mixture affect the YIELD and REACTION RATE?
| YIELD | REACTION RATE | |
| A | Increases | Increases |
| B | Remains the same | Remains the same |
| C | Remains the same | Increases |
| D | Decreases | Increases |
(2)
1.6 A hypothetical reaction reaches equilibrium at a certain temperature in a closed container according to the following balanced equation:
A(g) + 2B(g) ⇌ 3C(s) ΔH < 0
Which ONE of the following changes to the equilibrium conditions will result in an INCREASE in the equilibrium constant, Kc?
- Increase in temperature
- Decrease in temperature
- Increase in pressure at constant temperature
- Decrease in pressure at constant temperature (2)
1.7 A hydrochloric acid solution, HCℓ(aq), and an acetic acid solution, CH3COOH(aq), of EQUAL CONCENTRATIONS are compared.
How do the H3O+(aq) concentration of HCℓ(aq) and the pH of HCℓ(aq) compare to that of CH3COOH(aq)?
| [H3O+] of HCℓ(aq) | pH of HCℓ(aq) | |
| A | Higher than | Higher than |
| B | Higher than | Lower than |
| C | Equal to | Equal to |
| D | Higher than | Equal to |
(2)
1.8 Two hypothetical half-reactions and their respective reduction potentials are shown below:
B+(aq) + e- ⇌ B(s) Eθ = -1,5 V
A2+(aq) + 2e- ⇌ A(s) Eθ = 2,5 V
A galvanic cell is set up using the above substances.
Which ONE of the following statements is CORRECT for this galvanic cell?
- B(s) is the reducing agent.
- A(s) is the oxidising agent.
- The mass of B(s) will increase.
- The mass of A(s) will decrease. (2)
1.9 In an electrolytic cell …
- the anode is the positive electrode.
- oxidation takes place at the cathode.
- electrons flow from the cathode to the anode.
- the mass of the anode increases. (2)
1.10 Which ONE of the following is used as a catalyst in the Ostwald process?
- Iron
- Copper
- Platinum
- Vanadium (V) oxide (2)
[20]
QUESTION 2 (Start on a new page.)
2.1 The IUPAC name of an organic compound is 4,4-dimethylpent-2-yne.
2.1.1 Write down the GENERAL FORMULA of the homologous series to which this compound belongs. (1)
2.1.2 Write down the STRUCTURAL formula of this compound. (3)
2.2 The organic compound below has one positional isomer and one functional isomer.
2.2.1 Define the term positional isomer. (2)
For this compound, write down the:
2.2.2 IUPAC name of its POSITIONAL isomer (2)
2.2.3 Structural formula of its FUNCTIONAL isomer (2)
2.3 Consider the condensed structural formula of an organic compound below.![]()
2.3.1 Is this a PRIMARY, SECONDARY or TERTIARY alcohol?
Give a reason for the answer. (2)
2.3.2 Write down the IUPAC name of the above compound. (2)
2.3.3 Write down the IUPAC name of the MAJOR ORGANIC PRODUCT formed when this compound undergoes an elimination reaction. (2)
[16]
QUESTION 3 (Start on a new page.)
The boiling points of five organic compounds (P, Q, R, S and T) are studied.
| COMPOUND | IUPAC NAME |
| P | Pentanal |
| Q | 2,2-dimethylbutane |
| R | 3-methylpentane |
| S | Hexane |
| T | Pentan-1-ol |
3.1 Define the term boiling point. (2)
The boiling points of compounds Q, R and S are compared.
3.2 Give a reason why this is a fair comparison. (1)
The boiling points of Q, R and S are given below (NOT necessarily in the correct order).
| 55 °C | 49,7 °C | 68 °C |
3.3 Which ONE of the three boiling points is most likely the boiling point of compound R? Explain the answer. (4)
3.4 A mixture of equal amounts of P and T is placed in a flask and heated to a temperature below their boiling points. Assume that no reaction or condensation takes place. The vapour produced is collected in a syringe.
3.4.1 Which compound (P or T) will be present in a greater amount in the SYRINGE? (2)
3.4.2 Explain the answer to QUESTION 3.4.1 by referring to the TYPES and STRENGTHS of intermolecular forces. (3)
[12]
QUESTION 4 (Start on a new page.)
The flow diagram below shows how compound A can be used to prepare other organic compounds. The numbers I, II, III and IV represent different organic reactions.
Use the information in the flow diagram to answer the following questions.
4.1 Name the homologous series to which compound A belongs. (1)
4.2 Write down the TYPE of reaction represented by:
4.2.1 I (1)
4.2.2 III (1)
4.2.3 IV (1)
4.3 Consider reaction III. Write down the:
4.3.1 TWO reaction conditions for this reaction (2)
4.3.2 IUPAC name of the primary alcohol that is formed (2)
4.4 Draw the STRUCTURAL FORMULA for compound B. (2)
4.5 Consider reaction IV. Write down the:
4.5.1 Structural formula of organic compound C (2)
4.5.2 NAME or FORMULA of the catalyst that is used (1)
[13]
QUESTION 5 (Start on a new page.)
The calcium carbonate (CaCO3) in antacid tablets reacts with dilute hydrochloric acid
(HCℓ) according to the following balanced equation:
CaCO3(s) + 2HCℓ(aq) → CaCℓ2(aq) + CO2(g) + H2O(ℓ) H < 0
5.1 Is the above reaction EXOTHERMIC or ENDOTHERMIC? Give a reason for the answer. (2)
An antacid tablet of mass 2 g is placed in HCℓ(aq). After 30 s the mass of the tablet was found to be 0,25 g.
5.2 Calculate the average rate (in g∙s-1) of the above reaction. (3)
The antacid tablet contains 40% calcium carbonate. Another antacid tablet of mass 2 g is allowed to react completely with HCℓ(aq).
5.3 Calculate the volume of carbon dioxide, CO2(g) that will be collected at STP.
Assume that all the CO2(g) produced is from the calcium carbonate. (5)
The reaction rate of similar antacid tablets with excess HCℓ(aq) of concentration 0,1 mol∙dm-3 at DIFFERENT TEMPERATURES is measured. The graph below was obtained.
Use the information in the graph to answer the following questions.
5.4 Write down ONE controlled variable for this investigation. (1)
5.5 Write down a conclusion that can be made from the graph. (2)
5.6 Use the collision theory to fully explain the answer to QUESTION 5.5 (3)
5.7 Redraw the graph above in the ANSWER BOOK. On the same set of axes, sketch the curve that will be obtained if HCℓ(aq) of concentration 0,2 mol∙dm-3 is now used. Label this curve Y. (2)
[18]
QUESTION 6 (Start on a new page.)
Initially 60,8 g pure carbon dioxide, CO2(g), is reacted with carbon, C(s), in a sealed container of volume 3dm3. The reaction reaches equilibrium at temperature T according to the following balanced equation:
C(s) + CO2(g) ⇌ 2CO(g)
6.1 Define the term chemical equilibrium. (2)
6.2 At equilibrium it is found that the concentration of the carbon dioxide is 0,054 mol∙dm-3.
Calculate the:
6.2.1 Equilibrium constant, KC, for this reaction at temperature T (7)
6.2.2 Minimum mass of C(s) that must be present in the container to obtain this equilibrium (3)
6.3 How will EACH of the following changes affect the AMOUNT of CO(g) at equilibrium?
Choose from INCREASES, DECREASES or REMAINS THE SAME.
6.3.1 More carbon is added to the container (1)
6.3.2 The pressure is increased by reducing the volume of the container at constant temperature.
Use Le Chatelier's principle to explain the answer. (3)
6.4 The table below shows the percentages of CO2(g) and CO(g) in the container at different temperatures.
| TEMPERATURE (°C) | % CO2(g) | % CO(g) |
| 827 | 6.23 | 93.77 |
| 950 | 1.32 | 98.68 |
| 1050 | 0.37 | 99.63 |
| 1200 | 0.06 | 99.94 |
6.4.1 Is the reaction EXOTHERMIC or ENDOTHERMIC?
Refer to the data in the table and explain the answer. (3)
6.4.2 Use the information in the table to determine temperature T. Show clearly how you arrived at the answer. (3)
[22]
QUESTION 7 (Start on a new page.)
A hydrogen bromide solution, HBr(aq), reacts with water according to the following
balanced chemical equation:
HBr(aq) + H2O(ℓ) ⇌ Br -(aq) + H3O+(aq)
The Ka value of HBr(aq) at 25 °C is 1 x 109.
7.1 Is hydrogen bromide a STRONG ACID or a WEAK ACID? Give a reason for the answer.(2)
7.2 Write down the FORMULAE of the TWO bases in the above reaction. (2)
7.3 HBr(aq) reacts with Zn(OH)2(s) according to the following balanced equation:
Zn(OH)2(s) + 2HBr(aq) → ZnBr2(aq) + 2H2O(ℓ)
An unknown quantity of Zn(OH)2(s) is reacted with 90 cm3 of HBr(aq) in a flask. (Assume that the volume of the solution does not change during the reaction.)
The EXCESS HBr(aq) is then neutralised by 16,5 cm3 of NaOH(aq) of concentration 0,5 mol·dm-3. The balanced equation for the reaction is:
HBr(aq) + NaOH(aq) → NaBr(aq) + H2O(ℓ)
7.3.1 Calculate the pH of the HBr solution remaining in the flask AFTER the reaction with Zn(OH)2(s). (7)
7.3.2 Calculate the mass of Zn(OH)2(s) INITIALLY present in the flask if the initial concentration of HBr(aq) was 0,45 mol∙dm-3. (6)
[17]
QUESTION 8 (Start on a new page.)
A standard electrochemical cell is set up using two standard half-cells, as shown in the diagram below.
8.1 State the energy conversion that takes place in this cell. (1)
8.2 What is the function of component Q? (1)
X is a metal. A voltmeter connected across the cell initially registers 1,49 V.
8.3 Use a calculation to identify metal X. (5)
8.4 Write down the NAME or FORMULA of the reducing agent. (1)
8.5 The reading on the voltmeter becomes ZERO after this cell operates for several hours.
8.5.1 Give a reason for this reading by referring to the rates of oxidation and reduction half-reactions taking place in the cell. (1)
A silver nitrate solution, AgNO3(aq), is NOW added to the chlorine half-cell and a precipitate forms.
8.5.2 How will the reading on the voltmeter be affected?
(Choose from INCREASES, DECREASES or REMAINS the same) (1)
8.5.3 Use Le Chatelier's principle to explain the answer to QUESTION 8.5.2. (2)
[12]
QUESTION 9 (Start on a new page.)
Chlorine is produced industrially by the electrolysis of a concentrated sodium chloride solution, NaCℓ(aq).
The balanced equation for the net (overall) cell reaction is as follows:
2NaCℓ(aq) + 2H2O(ℓ) → H2(g) + 2NaOH(aq) + Cℓ2(g)
9.1 Define the term electrolysis. (2)
9.2 For the above reaction, write down the:
9.2.1 Half-reaction that takes place at the cathode (2)
9.2.2 NAME or FORMULA of the oxidising agent (1)
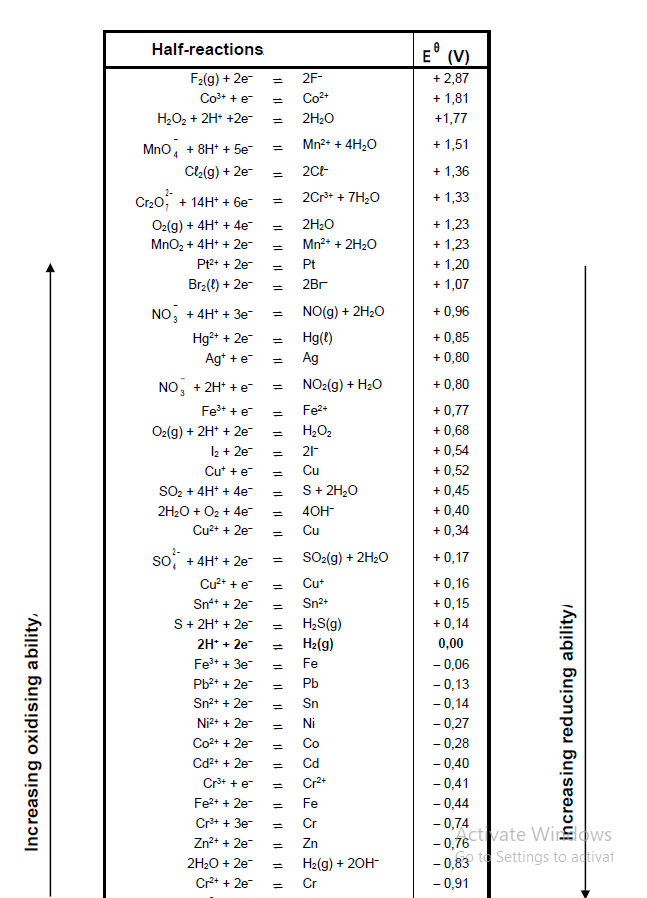
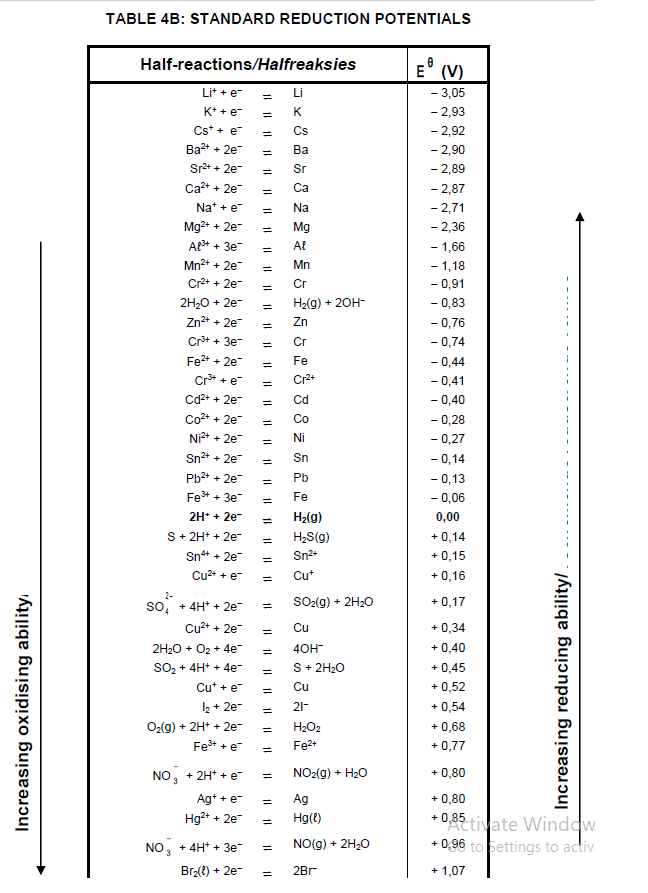
9.3 Refer to the Table of Standard Reduction Potentials to explain why sodium ions are not reduced during this process. (3)
[8]
QUESTION 10 (Start on a new page.)
The flow diagram below shows the processes involved in the industrial preparation of an ammonium fertiliser.
10.1 Write down the NAME or FORMULA of:
10.1.1 Gas X (1)
10.1.2 Gas Y (1)
10.1.3 Acid Q (1)
10.2 Write down the:
10.2.1 TYPE of chemical reaction that converts compound P into gas Y (1)
10.2.2 Balanced equation for the reaction between compound P and acid Q (3)
10.3 Two separate bags of fertilisers are labelled as follows:
10.3.1 What do the numbers (21) and (27) on the labels represent? (1)
10.3.2 Determine, by means of calculations, which bag (A or B) contains a greater mass of phosphorous. (4)
[12]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 2(CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | e -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m | n = N NA |
| c = n V or c = m MV | n = V Vm |
| caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | |
| Eθ cell = Eθ cathode – Eθ anode Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |
TABLE 4A: STANDARD REDUCTION POTENTIALS