PHYSICAL SCIENCES CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - AMENDED SENIOR CERTIFICATE EXAM PAST PAPERS AND MEMOS MAY/JUNE 2016
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPhysical Sciences (chemistry)
Paper Two (P2)
Grade 12
Amended Senior Certificate Exam
Past Papers And Memos May/june 2016
MEMORANDUM
QUESTION 1
1.1 A ✓✓ (2)
1.2 B ✓✓ (2)
1.3 B ✓✓ (2)
1.4 C ✓✓ (2)
1.5 B ✓✓ (2)
1.6 D ✓✓ (2)
1.7 C ✓✓ (2)
1.8 B ✓✓ (2)
1.9 A ✓✓ (2)
1.10 C ✓✓ (2)
[20]
QUESTION 2
2.1
2.1.1 E ✓ (Accept: methyl propanoate) (1)
2.1.2 C ✓ (Accept: butan-1-ol) (1)
2.1.3 D ✓ (Accept: 2,2-dimethylpropane) (1)
2.2
2.2.1 Pent-2✓-yne✓ OR 2✓-pentyne✓ (2)
Marking criteria for 2.2.1
|
2.2.2 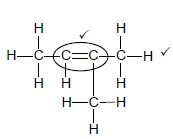 (2)
(2)
Marking criteria for 2.2.2:
|
2.2.3 2-methylbut-1-ene OR 3-methylbut-1-ene
Accept 2-methyl-1-butene (3)
Marking criteria:
|
2.3
2.3.1 Esters ✓ (1)
2.3.2 Sulphuric acid/H2SO4 ✓ (1)
2.3.3 Methyl✓ propanoate ✓ (2)
Marking criteria:
|
[14]
QUESTION 3
3.1 The temperature at which the vapour pressure equals the atmospheric pressure (external pressure). ✓✓ (2 marks or no marks)
3.2
Criteria for conclusion: | |
Dependent and independent variables correctly identified. | ✓ |
Relationship between the independent and dependent variables correctly stated. | ✓ |
Examples:
- Boiling point increases with increase in number of (C) atoms/chain length/ molecular size/molecular mass.
- Boiling point decreases with decrease in number of C atoms/chain length/ molecular size/molecular mass.
- Boiling point is proportional to number of C atoms/chain length/molecular size/molecular mass. (2)
IF:
|
3.3
3.3.1 P ✓ (1)
3.3.2 R ✓ (1)
3.4 ∙
- Between alkane molecules are London forces/dispersion forces/induced dipole forces. ✓
- In addition to London forces and dipole-dipole forces each alcohol molecule has (one site) for hydrogen bonding. ✓
- In addition to London forces and dipole-dipole forces each carboxylic acid molecule has two sites for hydrogen bonding. ✓ (Accept: more sites)
- Intermolecular forces in carboxylic acids are stronger than intermolecular forces in alkanes and alcohols./Intermolecular forces between alkane and alcohol molecules are weaker than intermolecular forces between carboxylic acid molecules.✓
- More energy is needed to overcome/break intermolecular forces in carboxylic acids than in the other two compounds. ✓ (5)
[11]
QUESTION 4
4.1
4.1.1 Addition/Hydrogenation ✓ (1)
4.1.2 Elimination/Dehydrohalogenation/Dehydrobromination ✓ (1)
4.1.3 Substitution/Halogenation/Bromination ✓ (1)
4.2
4.2.1 Pt/Ni/Pd/platinum/nickel/palladium ✓ (1)
4.2.2 H2SO4/H3PO4/sulphuric acid/phosphoric acid ✓ (1)
4.2.3 Hydration ✓ (1)
4.2.4 2✓-bromopropane ✓ (2)
Marking criteria:
|
4.3 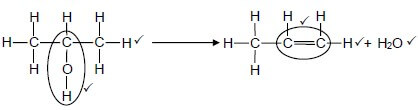 (5)
(5)
| Notes: Whole structure of alkene correct: ✓✓ Only functional group correc: ✓ |
Notes:
|
4.4 ∙
- Higher temperature ✓
- Concentrated base/Base dissolved in ethanol ✓ (2)
[15]
QUESTION 5
5.1 ANY TWO:
- Temperature (of reaction mixture)✓
- (Addition of a) catalyst ✓
- Concentration (of reactants) (2)
5.2 Sulphur/S ✓ (1)
5.3 Water is used to dilute/change the concentration (of the Na2S2O3(aq)) ✓ (1)
5.4
Criteria for investigative question: | |
The dependent and independent variables are stated correctly. | ✓ |
Asks a question about the relationship between dependent and independent variables.. | ✓ |
Dependent variable: rate (of reaction)/(reaction rate)
Independent variable: concentration
Examples:
- What is the relationship between concentration and reaction rate?
- How does the reaction rate change with change in concentration? (2)
5.5 A ✓ (1)
5.6 Experiment B:
- The concentration of Na2S2O3(aq) is higher./More Na2S2O3 particles per unit volume. ✓Accept: higher volume of Na2S2O3(aq) is used
- More particles with correct orientation ✓
- More effective collisions per unit time / Higher frequency of effective collisions. ✓
OR
Experiment D:
- The concentration of Na2S2O3(aq) is lower./Less Na2S2O3 particles per unit volume. ✓
- Less particles with correct orientation.✓
- Less effective collisions per unit time./Lower frequency of effective collisions. ✓ (3)
5.7
Marking guidelines for Option 1 and 2:
|
Marking guidelines for Option 3 and 4:
|
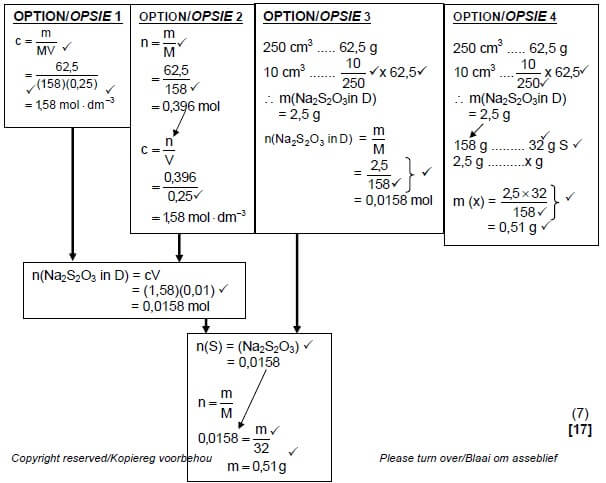
QUESTION 6
6.1 Reversible reaction ✓ (1)
6.2 Endothermic ✓ → ∆H is positive./∆H > 0/(Net) energy is absorbed./More energy is absorbed than released/Energy of product > energy of reactant. ✓ (2)
6.3 Larger than ✓ → Kc > 1 ✓ (2)
6.4 (9)
CALCULATIONS USING NUMBER OF MOLES
|
OPTION 1
n = m/M
= 168/28
= 6 mol
CO2 | CO | REMARKS | |
Initial quantity (mol) | x | 0 | |
Change (mol) | 3 | 6 ✓ | ratio ✓ |
Quantity at equilibrium (mol)/ | x – 3 ✓ | 6 | |
Equilibrium concentration (mol·dm-3) | x − 3 | 3 | Divide by 2 ✓ |
Kc = [CO]2
[CO2]✓
14 ✓ = (3)2
x − 3
2
∴x = 4,29 mol ✓
| No KC expression, correct substitution: Max 8/9 |
| Wrong KC expression: 6/9 |
OPTION 2
n = m/M c = n/v
= 168/28 = 6/2 (divide by 2)
= 6 mol = 3 mol.dm-3
CO2 | CO | ||
Initial concentration (mol·dm-3) | x | 0 | |
Change (mol·dm-3) | 1,5 | 3 ✓ | ratio✓ |
Equilibrium concentration (mol·dm-3) | x −1,5 ✓ | 3 |
Kc = [CO]2
[CO2]✓
14 ✓ = (3)2
x −1,5
∴x = 2,14 mol·dm-3
n(CO2) = cV
= (2,14)(2)
= 4,29 mol ✓
| No KC expression, correct substitution: Max 8/9 |
| Wrong KC expression: 6/9 |
OPTION 3
n = m/M
= 168/28
= 6 mol
CO2 | CO | ||
Initial quantity (mol) | 4,28✓ | 0 | |
Change (mol) | 3 | 6 | ratio ✔ |
Quantity at equilibrium (mol)/ | 1,28 ✓ | 6✔ | |
Equilibrium concentration (mol·dm-3) | 0,64 | 3 | multiply by 2 ✔ |
Kc = [CO]2
[CO2]✓
14 ✓ = (3)2
[CO2]✓
∴ [CO2] = 0,64 mol·dm-3
| No KC expression, correct substitution: Max 8/9 |
| Wrong KC expression: 6/9 |
6.5
6.5.1 Remains the same ✓ (1)
6.5.2 Decreases✓ (1)
6.5.3 Increases✓ (1)
[17]
QUESTION 7
7.1
7.1.1 An acid is a proton/ H+ donor. ✓✓ NOTE: not H3O+ (2 or/of 0) (2)
7.1.2 H2O ✓
H2CO3 ✓ (2)
7.1.3 H2O ✓ OR/OF HCO-3 (1)
7.2
7.2.1
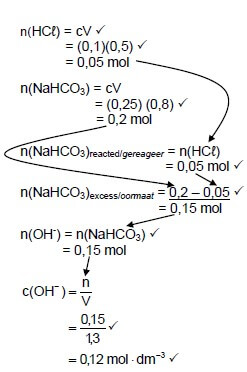 (8)
(8)
Marking guidelines:
|
7.2.2 POSITIVE MARKING FROM QUESTION 7.2.1 (4)
OPTION 1 | OPTION 2 pH + pOH = 14 |
[17]
QUESTION 8
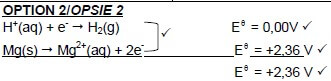
8.1 Electrons are transferred. ✓ OR/OF The oxidation number of Mg/H changes. OR/OF Mg is oxidised / H+ is reduced. (1)
8.2 H+ ions/HCℓ/H+(aq)/HCℓ(aq) ✓ (1)
8.3 Ag is a weaker reducing agent ✓(than H2) and will not be oxidised ✓to Ag+ ✓ OR/OF H2 is a stronger reducing agent ✓ (than Ag) and will be oxidised ✓ to H+.✓ (3)
8.4 Electrode/Conductor of electrons (in hydrogen half-cell) ✓ (1)
8.5
8.5.1 Chemical energy to electrical energy ✓ (1)
8.5.2 Provides path for movement of ions./Completes the circuit./Ensures electrical neutrality in cell. ✓ (1)
8.5.3 2H+ + 2e- → H2 ✓✓ (2)
Notes H2 ← 2H+ + 2e- (0/2 ) 2H+ + 2e ← H2 (0/2 ) |
8.5.4 Mg(s) | Mg2+(aq) || H+(aq) | H2(g) | Pt ✓ ✓ ✓ OR/OF Mg(s) | Mg2+(1 mol·dm-3) || H+(1 mol·dm-3) | H2(g) | Pt
Accept
Mg | Mg2+ || H+ | H2 | Pt (3)
8.6 (4)
OPTION 1 | Notes
|
OPTION 2
| |
8.7 Increases✓ (1)
[18]
QUESTION 9
9.1
9.1.1 Electrolyte✓ (1)
9.1.2 Electrolytic (cell) ✓
Electrolysis 0/1 (1)
9.2 A to/na B ✓ (1)
9.3
9.3.1 B ✓(1)
9.3.2 A ✓(1)
9.4 Decreases ✓ Copper (Cu) is oxidised to Cu2+/Oxidation takes place at A/Electrons are lost. ✓
[7]
QUESTION 10
10.1
10.1.1 Air ✓ (1)
10.1.2 Natural gas/methane/oil/coal ✓ (1)
10.1.3 Sulphur/iron pyrite/Iron sulphide ✓ (1)
10.2
10.2.1 Haber ✓ (1)
10.2.2 Ammonia✓ (1)
10.2.3 H2SO4 ✓ (1)
10.2.4 SO3+ H2SO4 ✓→ H2S2O7 ✓ Bal. ✓ (3)
Notes:
|
10.3
10.3.1 %N[NH4NO3] = 28/80 ✓ x 100 = 35%
%N[(NH4)2SO4] = 28/132 ✓ x 100 = 21,21%
Ammonium nitrate (has the highest percentage of nitrogen) ✓ (4)
10.3.2 Ostwald (process) ✓ (1)
[14]
TOTAL: 150