Physical Sciences P2 Grade 12 Questions - NSC Exams Past Papers and Memos September 2019 Preparatory Examinations
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub-questions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 The reaction represented by the balanced equation below occurs in the second step of the contact process.
2SO2(g) + O2(g) ⇌ 2SO3(g)
The catalyst used in the reaction above, is …
- nickel.
- platinum.
- iron (II) oxide.
- vanadium pentoxide. (2)
1.2 Which ONE of the following homologous series is saturated hydrocarbons?
- Esters
- Alkanes
- Alkenes
- Alkynes (2)
1.3 Which ONE of the following pairs of compounds are members of the same homologous series?
- C3H6 and C4H10
- CH4O and C2H4O2
- C2H4O2 and C3H6O2
- C3H6 and C4H6 (2)
1.4 In the flow diagram below, butane, C4H10, reacts to produce compound B in reaction 1. Compound B undergoes addition polymerisation to produce polyethene.
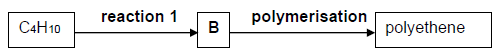
The name of the reaction represented by reaction 1, is …
- cracking.
- hydration.
- dehydration.
- dehydrohalogenation. (2)
1.5 The yield in a certain reversible reaction at equilibrium at temperature T and pressure P is 40%.
A catalyst is added to the reaction mixture at the start of the reaction and the reaction reaches equilibrium at the same temperature T and pressure P.
What effect will the addition of a catalyst have on the yield and rate of reaction?
| yield | reaction rate | |
| A | remains 40% | higher |
| B | remains 40% | Remains the same |
| C | higher than 40% | higher |
| D | higher than 40% | remains the same |
1.6 The graphs given below show how the vapour pressure of a secondary alcohol and a tertiary alcohol of equal molecular mass change with temperature.
Atmospheric pressure = 760 mmHg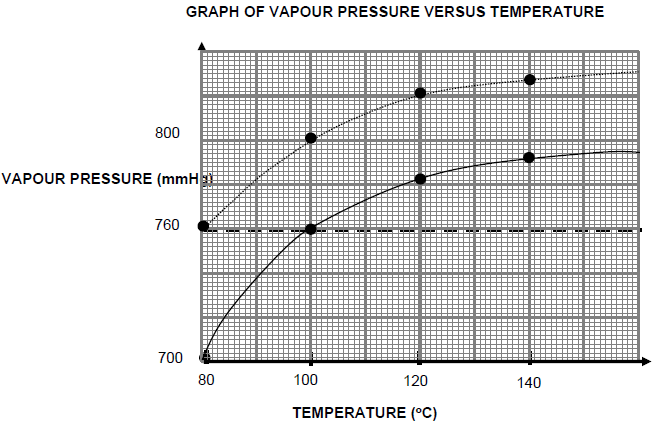
Which ONE of the following is the boiling point (in °C) of the secondary alcohol?
- 80
- 100
- 120
- 140 (2)
1.7 The following acid-base reactions occur spontaneously at the same temperature. All the solutions have the same concentration.
HPO42- (aq) + CO32- (aq) → PO43- (aq) + HCO3-(aq)
HPO42- (aq) + HSO4- (aq) → H2PO4-(aq) + SO42-(aq)
The dissociation constants (Kb values) are as follows:
K1 for HPO4-
K2 for CO32-
K3 for HSO4-
Which ONE of the following CORRECTLY shows the order of increasing Kb values?
- K1, K2, K3
- K3, K2, K1
- K2, K1, K3
- K3, K1, K2 (2)
1.8 Which ONE of the salts below produce an acidic solution when dissolved in water?
- Na2CO3
- NaCℓ
- NH4Cℓ
- KNO3 (2)
1.9 Which ONE of the following is the strongest reducing agent?
- Ni
- Cr2+
- Sn2+
- Ag (2)
1.10 An iron nail is electroplated with silver.
The half reaction taking place at the iron nail is given by:
- Fe2+ + 2e- → Fe
- Fe → Fe2+ + 2e-
- Ag → Ag+ + e-
- Ag+ + e- → Ag (2)
[20]
QUESTION 2 (Start on a new page.)
Three organic compounds (A, B and C) with different functional groups are given below.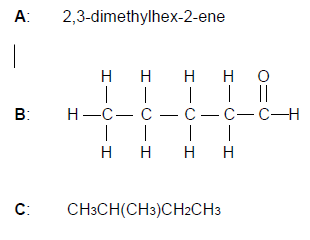
2.1 Write down TWO special properties of carbon which make it possible for carbon to form such a variety of organic compounds. (2)
2.2 Define the term functional group. (2)
2.3 Write down the:
2.3.1 Structural formula of compound A (2)
2.3.2 IUPAC name of compound B (2)
2.4 Compound C, pentane and a compound X are compounds that have the same molecular formula but different structural formulae.
Write down the:
2.4.1 Term used for the underlined phrase. (1)
2.4.2 Structural formula and IUPAC name of compound X (4)
[13]
QUESTION 3 (Start on a new page.)
The graph of the boiling point versus the number of carbon atoms for the first five STRAIGHT CHAIN alcohols and aldehydes is shown below.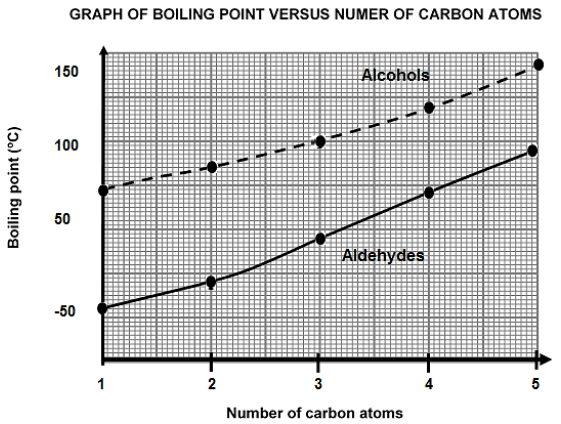
3.1 Define the term boiling point. (2)
3.2 Write down the IUPAC name of the alcohol with a boiling point of 100 °C. (2)
3.3 Explain fully why the curve for the alcohols is higher than that of the aldehydes. (5) The boiling points of carboxylic acids are generally HIGHER than those of their corresponding alcohols.
3.4 Explain the difference between the boiling points referring to the types of intermolecular forces present in each of these compounds. (3)
[12]
QUESTION 4 (Start on a new page.)
4.1 Consider the TWO reactions of haloalkanes with sodium hydroxide (NaOH) shown below.
- : CH3CH2CH2CH2Br

- : CH3CH2CHBrCH3

4.1.1 Which reaction (I or II) is classified as elimination reaction? (1) Write down:
4.1.2 The IUPAC name of the ORGANIC product formed in reaction I. (2)
4.1.3 A balanced equation for reaction II using structural formulae for the organic reagents. (5)
4.2 Consider the TWO organic compounds (A and B) shown below.
- : C4H8
- : C4H10
4.2.1 Which organic compound (A or B), will undergo addition reactions? Give a reason for the answer. (2)
4.2.2 Write down the NAME of an inorganic substance that reacts with compound A to produce compound B. (1)
4.3 Butanol reacts with organic compound Y in the presence of a concentrated inorganic acid to produce an ester. The equation shown below represents the reaction.
CH3CH2CH2CH2OH + Y → CH3CH2CH2CH2OOCCH2CH3 + H2O
Write down the:
4.3.1 Function of the concentrated inorganic acid in the reaction. (1)
4.3.2 Condensed structural formula of compound Y (2)
4.3.3 IUPAC name of the ester produced (2)
4.3.4 ONE piece of evidence that will indicate that an ester has been produced (1)
[17]
QUESTION 5 (Start on a new page.)
A group of learners use the reaction between magnesium and hydrochloric acid to measure the average rate at which hydrogen gas is produced. They add 10 cm3 of a 1 mol·dm-3 of HCℓ to 0,048 g magnesium powder in an Erlenmeyer flask at 20ºC.
The balanced equation for the reaction is: Mg(s) + 2HCℓ(aq) → MgCℓ2(aq) + H2(g)
The learners’ experimental results were plotted to produce graph A.
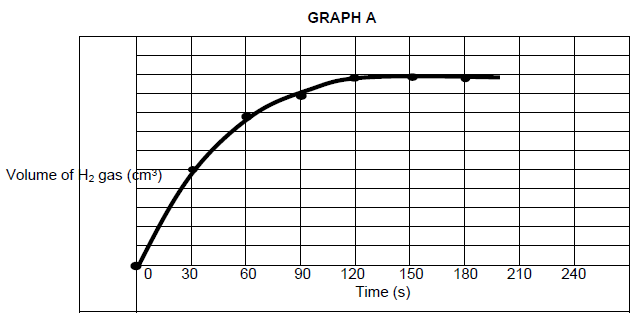
5.1 Define reaction rate. (2)
5.2 Calculate the volume of hydrogen gas produced in the first minute if the average rate of production of hydrogen gas is 0,67 cm3·s-1. (3)
5.3 How long (in seconds) does the reaction take to run to completion? Give a reason for the answer by referring to the gradient of the graph. (2)
5.4 Calculate the mass of the reactant left in the flask when the reaction is complete. (7)
5.5 When the concentration of hydrochloric acid is increased the learners observe that the rate of reaction increases. Use the collision theory to explain this observation. (2)
5.6 In another experiment the magnesium powder is replaced with an equal amount of zinc powder. Redraw the graph provided above in your ANSWER BOOK and sketch on the same axis the graph that would be obtained when zinc powder is used. (2)
[18]
QUESTION 6 (Start on a new page.)
The decomposition reaction of hydrogen iodide, HI represented by the balanced equation below reaches equilibrium in a closed container at 25ºC.
2HI(g) ⇌ H2(g) + I2(g) ∆H>0
6.1 How does the rate of the forward reaction compare to the rate of the reverse reaction at the following stages?
Choose from HIGHER THAN, LOWER THAN or EQUAL TO
6.1.1 At equilibrium (1)
6.1.2 Before the reaction reaches equilibrium for the first time? (1)
6.2 What effect will an increase in pressure, by decreasing the volume at constant temperature have, on the concentration of H2 at equilibrium?
Choose from INCREASES, DECREASES or REMAINS THE SAME (2)
6.3 The reaction is started by pumping a certain amount of hydrogen iodide, HI into an empty flask which is then sealed.
The equilibrium concentration of two of the substances at 25ºC was found to be:
[I2] = 0,026 mol·dm-3
[HI] = 0,72 mol·dm-3
When temperature of the equilibrium mixture is increased, the equilibrium position shifts and a new equilibrium is established at 448ºC. At the new equilibrium the concentration of hydrogen, H2 is found to be 0,084 mol·dm-3.
Calculate the equilibrium constant for the reaction at 448ºC. (7)
6.4 What effect will the increase in temperature, from 25ºC to 448ºC, have on the rate of the reverse reaction? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
[12]
QUESTION 7 (Start on a new page.)
7.1 Acid-base indicators are generally represented by the formula, HIn. The reaction of HIn with water can be represented by the following equation.
HIn + H2O ⇌ H3O+ + In-
colourless pink
Acid-base indicators are considered to be weak acids.
7.1.1 Define the term weak acid. (2)
7.1.2 Is H2O acting as an ACID or a BASE in the reaction? (1)
7.1.3 Write down the formula of the conjugate base of HIn. (1)
7.2 Vinegar is a solution of ethanoic acid, CH3COOH. A certain manufacturer of vinegar claims that the vinegar she sells contains 5,80 grams of ethanoic acid per 100 mℓ vinegar solution.
A group of learners used the apparatus shown below to test the claim by the manufacturer.
They titrated a dilute sample of vinegar against a standard sodium hydroxide solution (NaOH) of concentration 0,1 mol·dm-3 using HIn as the acid-base indicator.
The balanced equation for the reaction is given below:
NaOH(aq) + CH3COOH(aq) → CH3COONa(aq) + H2O(ℓ)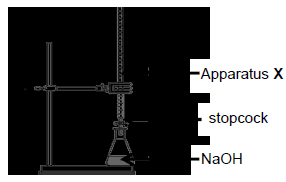
7.2.1 Write down the name of apparatus X. (1)
7.2.2 Use the equation in QUESTION 7.1 to determine the colour change that will take place at the end-point.
Choose from PINK TO COLOURLESS or COLOURLESS TO PINK.
Use Le Chatelier’s principle to explain the answer. (4)
7.2.3 Calculate the pH of the sodium hydroxide (NaOH) solution before titration. (4)
7.2.4 The dilute solution of vinegar used in the titration was obtained by adding 10 cm3 of vinegar to water and filling up with water to a volume of 100 cm3 of dilute vinegar solution.
During the titration 18 cm3 of sodium hydroxide solution of concentration 0,1 mol·dm-3 neutralises exactly 20 cm3 of the diluted vinegar.
Determine by calculation whether the manufacturer’s claim is TRUE or NOT. (8)
[21]
QUESTION 8 (Start on a new page.)
The diagram given below shows a galvanic cell set up under standard conditions.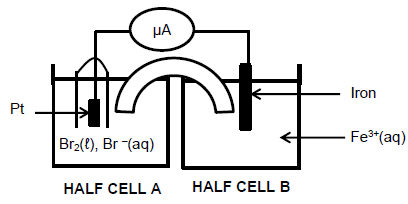
8.1 Write down TWO functions of the salt bridge. (2)
8.2 Which half-cell, A or B, contains the cathode? (1)
8.3 Write down the balanced equation for the overall (net) cell reaction. (3)
8.4 Calculate the initial EMF of this cell. (4)
8.5 The Br2|Br- half cell is now replaced with the I2|I- half-cell at standard conditions. Will the initial ammeter reading be HIGHER or LOWER when the I2|I- is used? Explain the answer by referring to the relative strengths of the oxidising agents involved. (3)
[13]
QUESTION 9 (Start on a new page.)
The diagram below show the apparatus used to demonstrate the electrolysis of concentrated copper(II) chloride (CuCℓ2) solution.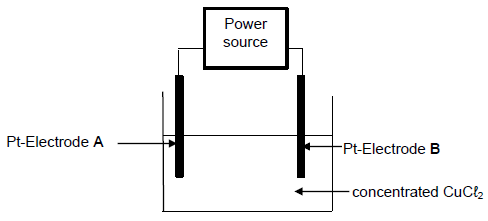
9.1 Write down the energy conversion which occurs in the cell above. (2)
9.2 Explain why an AC-power source is not suitable for this cell. (2)
A reddish-brown layer is observed on electrode A after the cell has been functioning for a while.
9.3 Write down the half reaction that occurs at electrode A.
The copper(II)chloride (CuCℓ2) solution is now replaced with a concentrated solution of sodium chloride (NaCℓ).
It is now observed that a gas is formed at electrode A. (2)
9.4 Write down the NAME of the gas that is formed at electrode A. (1)
9.5 Refer to the relative strengths of oxidising agents involved to explain why sodium (Na) metal does not form in this cell. (3)
[10]
QUESTION 10 (Start on a new page.)
The flow diagram below shows the industrial preparation of fertiliser Q.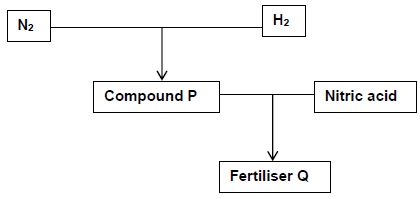
10.1 Write down:
10.1.1 The name of the process used to obtain nitrogen gas (N2) (1)
10.1.2 The name of compound P (2)
10.1.3 A balanced chemical equation for the production of fertiliser Q (3)
10.1.4 The name of the primary nutrient present in fertiliser Q (1)
10.2 Consider the three fertiliser bags shown below.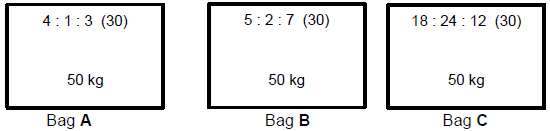
10.2.1 Which bag of fertiliser (A, B or C) is the most suitable for garden lawns?
Give a reason for the answer. (3)
10.2.2 Calculate mass of nitrogen in fertiliser bag C. (3)
10.3 Write down ONE negative impact of the overuse of fertilisers. (1)
[14]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,013 × 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
Charge on electron | e | -1,6 × 10-19 C |
Avogadro’s constant | NA | 6,02 × 1023 mol-1 |
TABLE 2: FORMULAE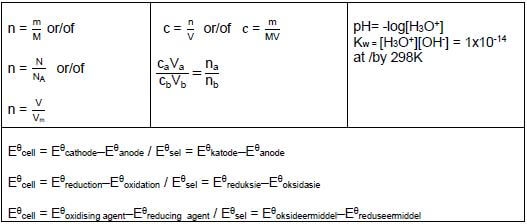
TABLE 3: THE PERIODIC TABLE OF ELEMENTS