Physical Sciences P2 Grade 12 Memorandum - NSC Exams Past Papers and Memos September 2019 Preparatory Examinations
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupQUESTIONS
QUESTION 1
1.1 D √√ (2)
1.2 B √√ (2)
1.3 C √√ (2)
1.4 A √√ (2)
1.5 A √√ (2)
1.6 B √√ (2)
1.7 D √√ (2)
1.8 C √√ (2)
1.9 B √√ (2)
1.10 D √√ (2) [20]
QUESTION 2
2.1
- Carbon can form four strong covalent bonds.
- Carbon can bond with itself to form long chains.
- Carbon can have single, double or triple bonds.
- Carbon can bond with hydrogen, oxygen, halogen and sulphur atoms.
(Any two √√) (2)
2.2. A bond or an atom or a group of atoms that determine(s) the physical and chemical properties of a group of organic compounds. (2)
2.3
2.3.1 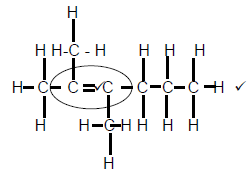
Marking criteria
- Whole structure correct 2/2
- Functional group correct ½ (2)
2.3.2 Pentanal (2)
2.4
2.4.1 (Structural) Isomer (1)
2.4.2 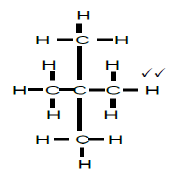
2,2-dimethylpropane
2,2-dimetielpropaan
- whole structure correct 2/2
- correct name 2/2
- if only propane correct 1/2
(4) [13]
QUESTION 3
3.1 The temperature at which the vapour pressure of a liquid equals the atmospheric/external pressure. (2)
3.2 propan -1-ol Accept 1-propanol (2)
3.3 Marking guidelines :
- For the same number of carbon atoms alcohols have higher boiling points
- Type of IMF in alcohol
- Type of IMF aldehydes
- Compare strength of IMFs
- Compare energy required
For the same number of carbon atoms alcohols have higher boiling points (than aldehydes)
Alcohols have hydrogen bonds while aldehydes have dipole-dipole forces.
Hydrogen bonds are stronger (than the dipole-dipole forces)
OR
Dipole-dipole forces are weaker (than hydrogen bonds)
More energy is required to overcome the Intermolecular forces/Hydrogen bonds/bonds in the alcohols. (5)
3.4
- Both (alcohol and carboxylic acid) have (london dispersion forces/induced dipole forces, dipole-dipole forces and) hydrogen bonds.
- Hydrogen bonds are stronger in carboxylic acids since they have two sites for hydrogen bonding (while alcohols have one site for hydrogen bonding) (3)
[12]
QUESTION 4
4.1
4.1.1 Reaction II. (1)
4.1.2 Butan-1-ol Accept 1-butanol (2)
4.1.3
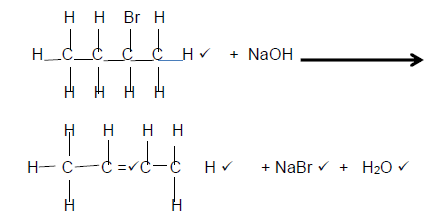 (5)
(5)
Marking Criteria
- Reactants
- Organic Product: Whole structure correct 2/2
Only functional group correct ½ - NaBr
- H2O
4.2
4.2.1 A/C4H8 . The molecule is unsaturated OR molecule has a double bond/multiple bond. (2)
4.2.2 Hydrogen (gas)(1)
4.3
4.3.1 It acts as a catalyst. (1)
4.3.2 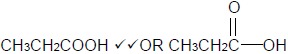 (2)
(2)
4.3.3 Butyl propanoate. (2)
4.3.4 (The distinct) ester smell. (1) [17]
QUESTION 5
5.1 The change in concentration (of reactants or products) per unit time.
OR
Change in amount OR mass OR volume (of product or reactant) per unit time
Rate of change in concentration/amount/number of moles/volume/mass. (2)
5.2
- 0,67 = v - 0
60−0
v = 40,2 cm3 (3)
5.3 120 s.
- The gradient of the graph is zero after 120 s. .
OR
The volume of H2 gas remains constant after 120 s.
OR
No more H2 gas is produced after 120 s.
OR/OF
One of the reactants is used up. (2)
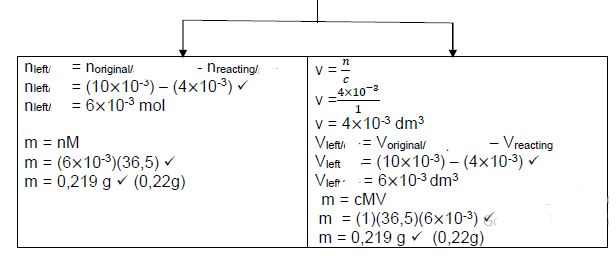
5.4
- Mg
n = ?
?
n = 0,048
24
n = 2 ×10-3 mol - HCℓ
c = ?
?
1 = ?
10×10−3
n = 10×10-3 mol
Mole: HCℓ : Mg
2 : 1
n (HCℓ) = 2 n (Mg)
n (HCℓ) = 2 (2 ×10-3 )
n (HCℓ) = 4×10-3 mol required
Therefore HCℓ is in excess
 (7)
(7)
5.5 Number of collisions with correct orientation increases
More effective collisions per unit time /Frequency of effective collisions increases (2)
5.6
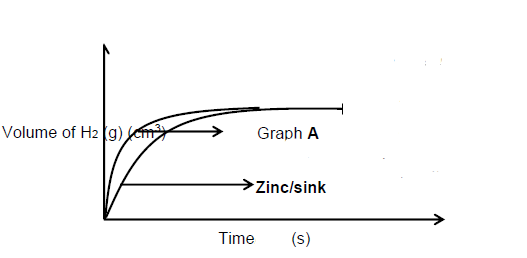
Marking guideline:
- Correct shape with graph of zinc below graph A
- Final volume the same
QUESTION 6
6.1 6.1.1 Equal to (1)
6.1.2 Higher than (1)
6.2 Increases (2)
6.3 Marking Criteria
- [H2] first equilibrium
- [I2] second equilibrium
- Ratio
- [HI] second equilibrium
- Kc expression
- Substitution into Kc expression
- Final answer
Range: 1,93 x 10-2 to 1,96 x 10-2
OPTION 1
CALCULATION USING CONCENTRATIONS
| 2HI | H2 | I2 | |
ci | 0,72 | 0,026ü | 0,026 |
Δc | 0,116 | 0,058 | 0,058 |
cequilibrium | 0,604ü | 0,084 ü | 0,084 |
Notes
- No Kc expression 6/7
- Wrong Kc expression 4/7
Kc = [H2].[I2]/[HI]2
= 0,0842/0,6042
= 1,93 x 10-2
Range: 1,93 x 10-2 to 1,96 x 10-2 (7)
OPTION 2
CALCULATION USING MOLES
2HI | H2 | I2 | |
ci | 0,72V | 0,026Vü | 00,026V |
Δc | 0,116 V | 0,058 | 0,058V |
cequilibrium | 0,604Vü | 0,084 V | 0,084Vü |
√ Ratio
Kc = [H2]·[I2]/[HI]2
= (0,084 V)2/(0,604 V)2
= 1,93 x 10-2
Range: 1,93 x 10-2 to 1,96 x 10-2
6.4 INCREASES (1) [12]
QUESTION 7
7.1
7.1.1 Weak acids ionise incompletely in water to form a low concentration of H3O+-ions. (2)
7.1.2 Base (1)
7.1.3 In- (1)
7.2
7.2.1 Burette (1)
7.2.2 PINK TO COLOURLESS
Adding ethanoic acid increases [H3O+]
The reaction that consumes H3O+ is favoured
The reverse reaction is favoured
OR
Equilibrium position shifts to the left (4)
7.2.3 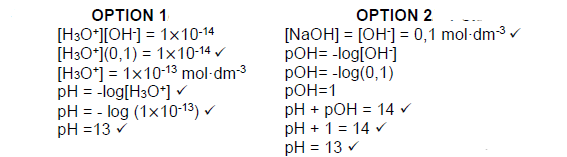 (4)
(4)
7.2.4 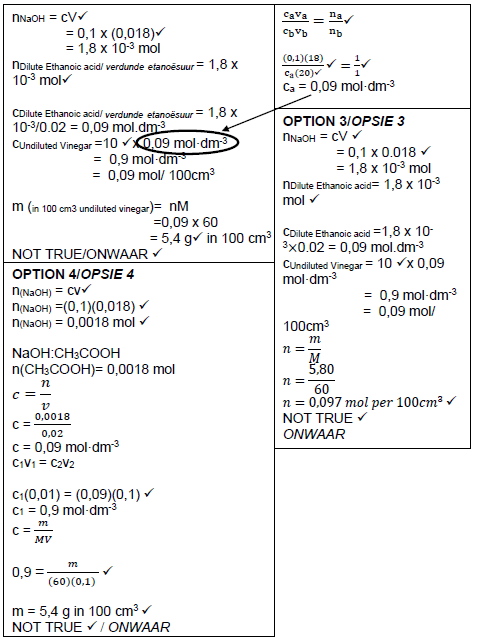
(8) [21]
QUESTION 8
8.1
- Completes the cell
- Keep the two half-cells neutral
- Keeps the reactants separate to allow for indirect transfer of electrons.
(Any TWO) (2)
8.2 Half-cell A (1)
8.3 2Fe + 3Br2 → 2Fe3+ + 6Br- Bal. (3)
Notes:
- Reactants Products Balancing
- Ignore double arrows
- Marking rule 6.3.10
8.4 Eθcell = Eθcathode–Eθanode
Eθcell = (1,07) – (- 0,06)
Eθcell = 1,13 V
Notes:
- Accept any other correct formula from the data sheet
- Any other formula using unconventional abbreviations e.g. Eo cell = EoOA – EoRA ¾ (4)
8.5 Lower Br2 is a stronger oxidising agent than I2. (3) [13]
QUESTION 9
9.1 Electrical energy is converted to chemical energy. (2)
9.2 When AC is used polarity of the electrodes will change continuously. OR DC ensures polarity of electrodes remains the same
(Any ONE/Enige EEN.) (2)
9.3 Cu2+ + 2e- → Cu (2)
9.4 Hydrogen (1)
9.5 H2O is a stronger oxidising agent than Na+ . H2O is reduced to H2 (3) [10]
QUESTION 10
10.1
10.1.1 Fractional distillation of liquid air (1)
10.1.2 Ammonia (2)
10.1.3 NH3 + HNO3 → NH4NO3 Bal. (3)
10.1.4 Nitrogen (1)
10.2 10.2.1 Bag A
(It contain the) highest ratio of nitrogen which promotes the growth of green leaves. (3)
10.2.2  (3)
(3)
10.3 Over fertilisation can lead to eutrophication.
Rootburn
(Any relevant answer) (1) [14]
TOTAL: 150