Technical Sciences Paper 2 Questions - Grade 12 September 2021 Preparatory Exams
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
- Write your FULL NAME and SURNAME in the appropriate spaces in the ANSWER BOOK.
- Answer ALL the questions.
- Start each question on a NEW page in the ANSWER BOOK.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Number the answers according to the numbering system used in this question paper.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1−1.5) in the ANSWER BOOK, for example 1.6 D.
1.1 A diode can convert …
- an electrical current to potential difference.
- potential difference to heat.
- an alternating current to a pulsating direct current.
- an electrical current to light. (2)
1.2 Which ONE of the following is a general formula of alkanes?
- C2nHn+2
- CnH2n
- CnH2n+2
- C2H2n (2)
1.3 Sharon, a Grade 12 Technical Sciences learner, left a solution of copper sulphate in a zinc container overnight. Early the next morning she noticed a brown insoluble substance coating the inside, around the sides and bottom of the zinc container. The container was corroded and some of the solution had leaked to the floor.
Which ONE of the following reactions took place inside the zinc container?
- Cu(s) + ZnSO4(aq) → CuSO4(aq) + Zn(s)
- Cu2+(aq) + ZnSO4(aq) → CuSO4(aq) + Zn(s)
- Zn2+(aq) + CuSO4(aq) → ZnSO4(aq) + Cu2+(aq)
- Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s) (2)
1.4 The diagram below represents a part of an electrochemical cell used for the electroplating of copper. The impure copper contains silver metal and zinc metal.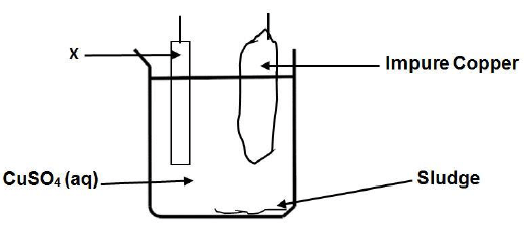
Which ONE of the following half-reactions will take place at electrode X?
- Ag+ + e- → Ag
- Cu → Cu2+ + 2e-
- Cu2+ + 2e- → Cu
- Zn2+ + 2e- → Zn (2)
1.5 Which ONE of the following reactions is spontaneous under standard conditions?
- Sn4+ + SO2 + 2H2O → Sn2+ + SO42- + 4H+
- I2 + 2Br - → 2I- + Br2
- 2H2O → O2 + 2H2
- 2Ag+ + Fe2+ → 2Ag + Fe3+ (2)
[10]
QUESTION 2 (Start on a NEW page.)
Give ONE word for each of the phrases given below.
2.1 An atom or group of atoms that determine the chemistry of a molecule (1)
2.2 A molecule that consists of a large number of atoms (1)
2.3 A material that has electrical conductivity between that of a conductor and an insulator(1)
2.4 The decomposition of a substance when an electric current passes through it (1)
[4]
QUESTION 3 (Start on a NEW page.)
Semiconductor devices such as diodes and transistors are widely used in modern electronics.
3.1 Define the term doping in words. (2)
3.2 Silicon is listed as an intrinsic semiconductor. Explain the meaning of an intrinsic semiconductor. (2)
3.3 A learner in a school laboratory adds boron to pure silicon to make it a better conductor of electricity.
3.3.1 Which type of semiconductor does this learner manufacture during the process above? (1)
3.3.2 A diode is a simple semiconductor device. How does a diode conduct electric current? (1)
[6]
QUESTION 4 (Start on a NEW page.)
Study the organic compounds represented by the letters A to G in the table below.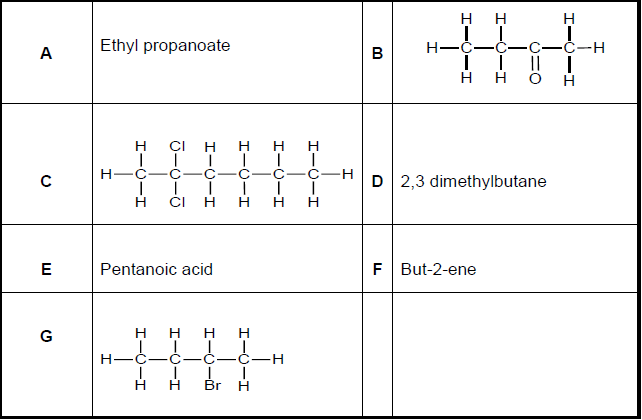
4.1 Write down the LETTER(S) that represent(s) each of following: (A compound may be used more than once.)
4.1.1 An alkyl halide (1)
4.1.2 An ester (1)
4.1.3 Two compounds that are structural isomers (2)
4.1.4 A ketone (1)
4.1.5 A compound containing a carboxyl group (1)
4.2 Write down the structural formula of compound D. (2)
4.3 Compound G is formed from compound F.
4.3.1 Name the type of reaction that produces compound G. (1)
4.3.2 Give the FORMULA of the other compound that reacted with compound F to form compound G. (1)
4.4 Give the IUPAC NAMES of the TWO compounds that will react to form compound A. (2)
[12]
QUESTION 5 (Start on a NEW page.)
A learner investigates the relationship between the structural isomers of pentane and their boiling points. The results obtained were recorded as shown below:
COMPOUND | MOLECULAR FORMULA | BOILING POINT (°C) | |
Pentane | C5H12 | 36 | |
2-methylbutane | C5H12 | 28 | |
2,2-dimethylpropane | C5H12 | 10 | |
5.1 Define the term homologous series. (2)
5.2 For this investigation, write down the conclusion that can be drawn from the above results. (1)
5.3 Refer to MOLECULAR STRUCTURE, INTERMOLECULAR FORCES and ENERGY needed, to explain your conclusion in QUESTION 5.2. (4)
5.4 What precaution should the learners take when carrying out this experiment? Give a reason for your answer. (2)
[9]
QUESTION 6 (Start on a NEW page.)
In industry, alkenes are used in the synthesis of a variety of organic compounds. The flow diagram below illustrates some of the many possible reactions.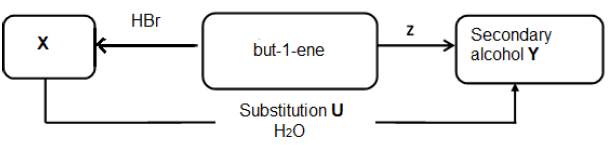
6.1 Use structural formulae to indicate the reaction for the formation of compound X. (4)
6.2 Name the type of reaction that takes place when but-1-ene is converted to compound X. (1)
6.3 Write down the structural formula and IUPAC name of the secondary alcohol Y that is formed. (3)
6.4 Name the type of substitution reaction U that takes place when compound X is converted to the secondary alcohol Y. (1)
6.5 With the aid of a catalyst, but-1-ene can be converted directly to the secondary alcohol, without the formation of the intermediate compound X.
6.5.1 Besides but-1-ene, write down the NAME of the other reactant needed for reaction Z. (1)
6.5.2 Write down the FORMULA of the catalyst that can be used. (1)
6.5.3 Name the type of reaction Z that will take place during this direct conversion. (1)
6.6 Give ONE industrial use of polyethylene. (1)
[13]
QUESTION 7 (Start on a NEW page.)
The diagram below represents an electrochemical cell used to decompose a concentrated copper(II) chloride solution using inactive electrodes.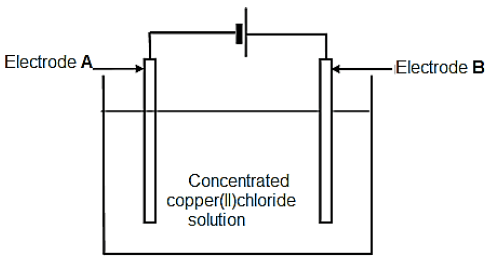
When the cell is functioning, a gas is released at electrode A, whilst electrode B is coated with a reddish brown layer.
7.1 Define the term electrolyte. (2)
7.2 What type of an electrochemical cell is represented above? (1)
7.3 Write down a half-reaction to explain the observation made at:
7.3.1 Electrode A (1)
7.3.2 Electrode B (1)
7.4 Write down the NAME of the gas formed while the cell is functioning. (1)
7.5 What energy conversion is taking place in this type of electrochemical cell? (1)
[7]
QUESTION 8 (Start on a NEW page.)
A Grade 12 Technical Sciences learner receives an assignment to arrange some metals according to their reducing ability. He sets up the electrochemical cell shown in the diagram below.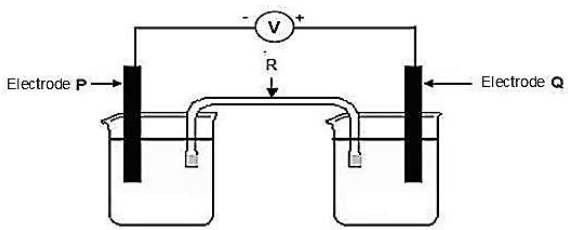
The learner uses different metals as electrodes. He records the following results:
COMBINATION | ELECTRODE P | ELECTRODE Q | VOLTMETER READING (V) |
1 | Chromium | Silver | +0,3 |
2 | Silver | Zinc | -0,7 |
3 | Zinc | Copper | 0,8 |
8.1 Write down the name of the component labelled R and give ONE function of this component. (2)
8.2 Write down the energy conversion that takes place in this cell when it is in operation. (1)
8.3 Give a possible reason why the voltmeter reading for the silver-zinc cell is negative. (1)
8.4 Consider the ZINC-CHROMIUM CELL represented by the notation below:
Zn(s)/Zn2+(aq, 1 mol·dm-3)//Cr3+ (aq, 1 mol·dm-3) /Cr(s)
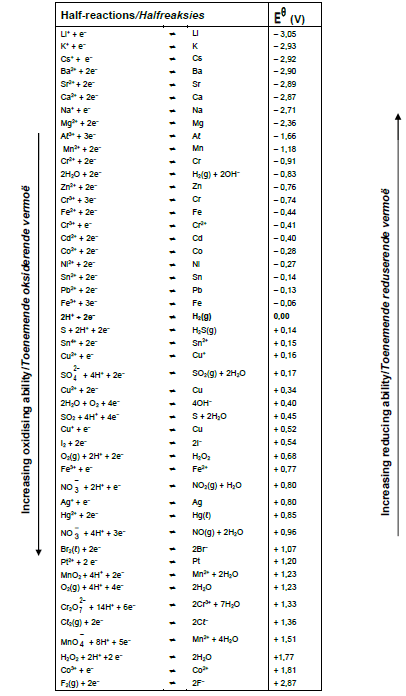
8.4.1 Use the table of Standard Reduction Potentials to determine the initial potential difference (emf) of this cell under standard conditions. (3)
8.4.2 How will the initial voltmeter reading be affected if the concentration of the electrolyte in the Zn(s)/Zn2+(aq) half-cell is increased? Write down only INCREASES, DECREASES, or REMAINS THE SAME. (1)
8.5 The learner notices that the measured value represented in the table and the calculated (emf) in QUESTION 8.4.1 differ. Give TWO possible reasons for this difference in values. (2)
8.6 Modern biodiesel fuel which is made by converting vegetable oils into compounds called fatty acid methyl esters, has its roots in research conducted in the 1930s in Belgium, but today’s biodiesel industry was not established in Europe until the late 1980s.
8.6.1 Give TWO other alternate energies except biodiesel in Technical Sciences. (2)
8.6.2 What is the environmental impact of using alternative energies? (1)
8.6.3 Give ONE disadvantage of using biodiesel as a fuel. (1)
[14]
TOTAL: 75
NATIONAL SENIOR CERTIFICATE
DATA FOR TECHNICAL SCIENCES GRADE 12 PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Avogadro’s constant | NA | 6,02 × 1023 mol-1 |
Molar gas constant | R | 8,31 J.K-1.mol-1 |
Standard pressure | pq | 1,013 × 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
TABLE 2: FORMULAE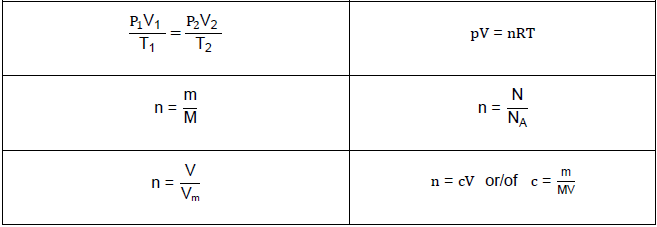
TABLE 3:THE PERIODIC TABLE OF ELEMENTS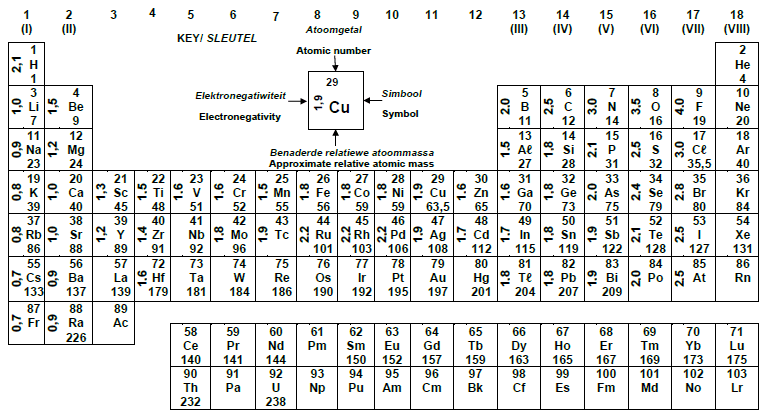
TABLE 4A: STANDARD REDUCTION POTENTIAL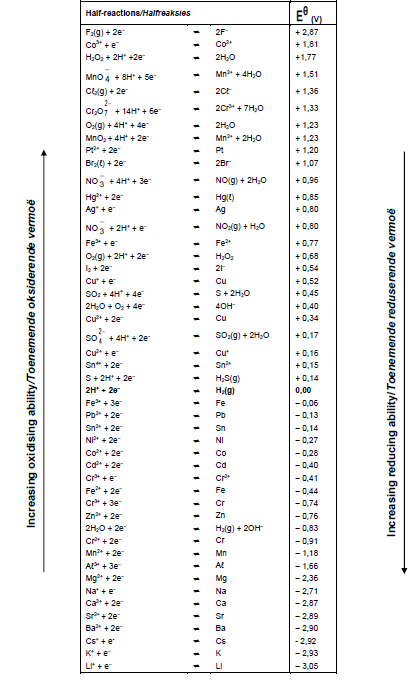
TABLE 4B:STANDARD REDUCTION POTENTIALS