TECHNICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC EXAMS PAST PAPERS AND MEMOS NOVEMBER 2020
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupTECHNICAL SCIENCES PAPER 2
GRADE 12
NOVEMBER 2020
NATIONAL SENIOR CERTIFICATE
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of NINE questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You are advised to use the attached DATA SHEETS.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions etc. where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 D.
1.1 Which ONE of the structural formulae below represents a secondary alcohol?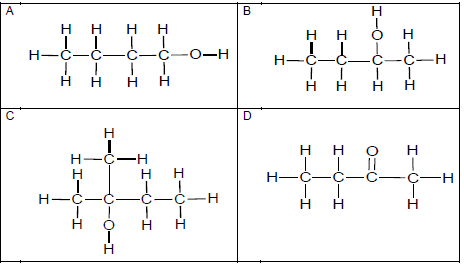
(2)
1.2 To which homologous series does dichloromethane belong?
- Alkanes
- Alcohols
- Haloalkanes
- Carboxylic acids (2)
1.3 In which ONE of the options below are the intermolecular forces arranged from the weakest to the strongest?
- Hydrogen bonds, dipole-dipole forces, London forces
- Hydrogen bonds, London forces, dipole-dipole forces
- Dipole-dipole forces, London forces, hydrogen bonds
- London forces, dipole-dipole forces, hydrogen bonds (2)
1.4 Alkenes react with hydrogen to form …
- alkanes.
- alcohols.
- aldehydes.
- alkynes. (2)
1.5 In an electrolytic cell the cations will migrate to the …
- cathode and undergo reduction.
- anode and undergo oxidation.
- cathode and undergo oxidation.
- anode and undergo reduction. (2)
1.6 In the cell notation of a galvanic cell, the double vertical lines (//) represent a/the …
- phase separator.
- solid electrode.
- gas electrode.
- salt bridge. (2)
1.7 Consider the statements below when an incident light ray is reflected from a flat surface.
- The angle of incidence is equal to the angle of reflection.
- The angle measured between the surface and the incident ray is the incidence angle.
- The incidence angle is the angle formed between the incident ray and the normal.
Which statement(s) is/are TRUE?
- (i) and (ii)
- (i) and (iii)
- (ii) only
- (i) only (2)
1.8 The diagram below shows light ray LM incident on a rectangular glass prism.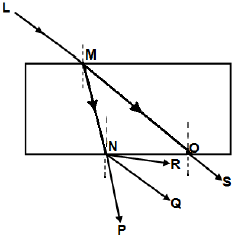
Which ONE of the following represents the CORRECT emergent ray?
- OS
- NR
- NQ
- NP (2)
1.9 During the dispersion of white light, which colour in the visible spectrum has the smallest angle of refraction?
- Green
- Violet
- Red
- Orange (2)
1.10 A learner used a lens (X) to focus the image of a well-illuminated faraway building on a screen (S), as shown in the diagram below.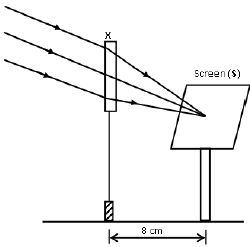
This lens is …
- concave of focal length 4 cm.
- convex of focal length 4 cm.
- concave of focal length 8 cm.
- convex of focal length 8 cm. (2)
[20]
QUESTION 2 (Start on a new page.)
Organic molecules can be classified into different homologous series that are identified by their functional groups.
2.1 Define the term functional group. (2)
2.2 Consider the organic molecules in the table below and answer the questions that follow.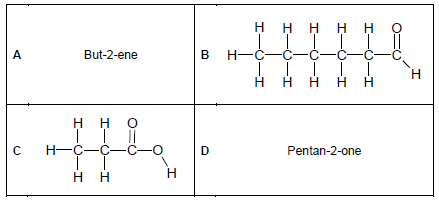
Write down the NAME of the homologous series of compounds represented by:
2.2.1 A (1)
2.2.2 B (1)
2.2.3 C (1)
2.3 Write down the IUPAC name of the organic compounds represented by:
2.3.1 B (2)
2.3.2 C (2)
2.4 Draw the structural formulae of the organic compounds represented by:
2.4.1 A (2)
2.4.2 D (2)
2.5 Draw the structural formulae of the ISOMER of the organic compounds represented by:
2.5.1 A (2)
2.5.2 C (2)
2.6 Identify the TYPE of isomer in:
2.6.1 QUESTION 2.5.1 (1)
2.6.2 QUESTION 2.5.2 (1)
2.7 Consider compound A.
2.7.1 Is compound A saturated or unsaturated? (1)
2.7.2 Explain the answer to QUESTION 2.7.1. (1)
[21]
QUESTION 3 (Start on a new page.)
The table below shows organic molecules with different molar masses and vapour pressures.
| COMPOUND | MOLAR MASS (g•mol-1) | VAPOUR PRESSURE (x 102Pa) |
| 1-propanol | 60 | 21.0 |
| 1-butanol | 74 | 6.2 |
| 1-pentanol | 88 | 2.2 |
3.1 Define the term vapour pressure. (2)
3.2 Use the table above to answer questions that follow.
3.2.1 Describe the trend in the vapour pressure of the compounds above. (1)
3.2.2 Explain the answer to QUESTION 3.2.1.
Refer to CHAIN LENGTH/MOLAR MASS, STRENGTH OF INTERMOLECULAR FORCES and ENERGY. (3)
3.3 Consider the graph below that indicates the boiling points of alkanes and aldehydes.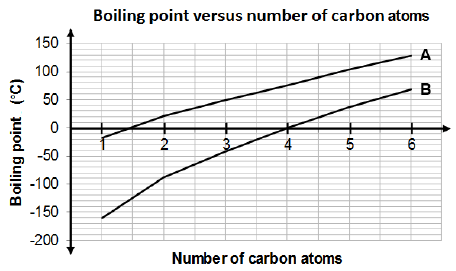
3.3.1 Identify the homologous series represented by graphs A and B respectively. (2)
3.3.2 Explain the difference in the boiling points of the two homologous series represented by graphs A and B. Refer to the TYPE and STRENGTH of the intermolecular forces. (3)
[11]
QUESTION 4 (Start on a new page.)
Use the flow diagram below to answer the questions that follow.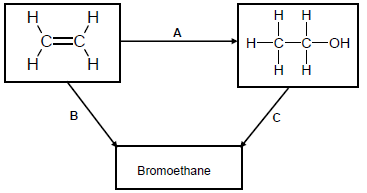
4.1 Write down the NAME or TYPE of reaction represented by the following letters:
4.1.1 A (1)
4.1.2 B (1)
4.1.3 C (1)
4.2 Apart from the alkene, another reactant and a catalyst are needed in reaction A. Write down the NAME or FORMULA of the:
4.2.1 Other reactant (1)
4.2.2 Catalyst (1)
4.3 Use STRUCTURAL FORMULAE to write down a balanced chemical equation for reaction B. (3)
4.4 Write TWO reaction conditions for reaction C. (2)
4.5 Use molecular formulae to write down a balanced equation for the reaction of the alkene in the flow diagram in excess oxygen. (3)
4.6 Write down the NAME of the organic compound in the flow diagram that can be used as a monomer of polythene. (1)
[14]
QUESTION 5 (Start on a new page.)
The diagram below represents the electrolysis of copper(ll) chloride.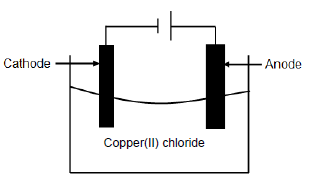
5.1 Define the term electrolysis. (2)
5.2 Is the electrolysis of copper(II) chloride a spontaneous or a non-spontaneous reaction? (1)
5.3 Explain the answer to QUESTION 5.2. (1)
5.4 Write down the FORMULA of an electrolyte used in the cell above. (1)
5.5 At which electrode will the following observations be made? Write down only ANODE or CATHODE.
5.5.1 Gas bubbles are formed. (1)
5.5.2 A brownish deposit is formed.(1)
5.6 Define the term reducing agent. (2)
5.7 For the cell above, write down the:
5.7.1 Oxidation half-reaction (2)
5.7.2 Reduction half-reaction (2)
5.7.3 Net reaction (2)
[15]
QUESTION 6 (Start on a new page.)
6.1 The cell notation Zn(s)|Zn2+ (aq)║Cu2+ (aq)|Cu(s) represents a galvanic cell operating under standard conditions.
6.1.1 Define the term galvanic cell. (2)
6.1.2 Draw a labelled diagram to represent the Zn-Cu cell. Show the direction of electron flow in the external circuit. (5)
6.1.3 Write down TWO standard conditions under which the Zn-Cu cell operates. (2)
6.1.4 To which half-cell do the anions in the salt bridge migrate? (1)
6.1.5 Explain the answer to QUESTION 6.1.4. (2)
6.2 The anode in the Zn-Cu cell is replaced by an unknown electrode, X. The voltmeter gives a reading of 2,00 V.
6.2.1 Identify electrode X by means of a calculation. (5)
6.2.2 Write down the half-reaction taking place at the anode.(2)
6.2.3 Which electrode will experience a decrease in mass? (1)
6.2.4 Explain the answer to QUESTION 6.2.3. (2)
[22]
QUESTION 7 (Start on a new page.)
7.1 The diagram below shows the reflection of a light ray that strikes a flat mirror with a 30° angle of incidence.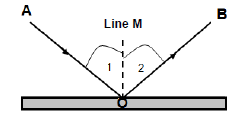
7.1.1 What is reflection? (2)
Use the diagram to write down the NAMES of:
7.1.2 Ray AO (1)
7.1.3 Ray OB (1)
7.1.4 Line M (1)
7.1.5 Angle 1 (1)
7.1.6 Angle 2 (1)
7.2 What is the magnitude of angle 2? (1)
7.3 How will the speed of light be affected when a light ray travels from air into water, perpendicular to the surface? (1)
7.4 A light ray enters the water from air at an angle.
7.4.1 Which medium, air or water, has the higher optical density? (1)
7.4.2 What will happen to the DIRECTION of the light ray as it enters the water? (1)
7.4.3 Write down the NAME of the phenomenon in QUESTION 7.4.2. (1)
7.5 A ray of light moves from water towards air and strikes the interfase (boundary) at an angle of incidence smaller than the critical angle.
7.5.1 Define the term critical angle. (2)
7.5.2 What observation will be made? (2)
The incident angle in QUESTION 7.5 is changed such that the ray undergoes total internal reflection.
7.5.3 Describe the phenomenon total internal reflection. (2)
7.5.4 Is the incident angle GREATER THAN or SMALLER THAN the critical angle? (1)
[19]
QUESTION 8 (Start on a new page.)
8.1 Define the term dispersion. (2)
8.2 Name any FOUR colours in visible light. (4)
8.3 State THREE properties of the image formed in a plane mirror. (3)
8.4 Redraw the diagrams below in the ANSWER BOOK and complete EACH to show the path of the ray after passing through the lens.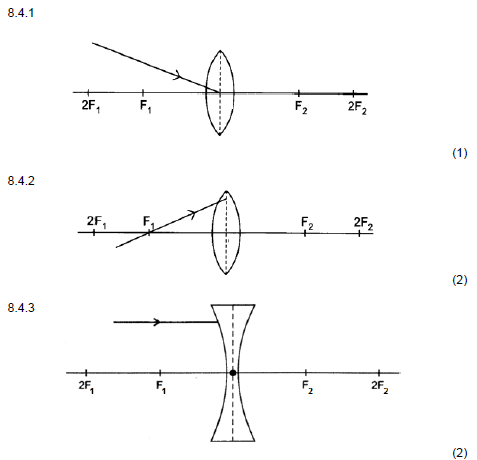
[14]
QUESTION 9 Start on a new page.)
9.1 Define an electromagnetic wave. (2)
9.2 Which property of radio waves makes it suitable to transmit a signal over long distances? (1)
9.3 What is a photon? (1)
9.4 Write down the NAME of the electromagnetic wave that is used:
9.4.1 To detect counterfeit notes (1)
9.4.2 To open and close automatic doors (1)
9.4.3 In navigation systems (1)
9.5 What is the relationship between the frequency of light and its wavelength? (2)
9.6 Calculate the energy of light with a wavelength of 4,06 x 10-11 m. (5)
[14]
TOTAL: 150
DATA FOR TECHNICAL SCIENCES GRADE 12 PAPER 2
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,01 x 105 Pa |
| Standard temperature | Tθ | 273 K |
| Speed of light in a vacuum | c | 3,0 x 108 m·s-1 |
| Planck's constant | h | 6,63 x 10-34 J·s |
TABLE 2: WAVES, SOUND AND LIGHT
| WAVES, SOUND AND LIGHT | |
| v = f λ | T = 1 f |
| Energy | E = hf |
TABLE 3: FORMULAE
| Eθcell= Eθcathode - Eθanode Eθcell = Eθreduction - Eθoxidation Eθcell= Eθoxidising agent - Eθreducing agent |