Physical Sciences Paper 2 Memorandum - Grade 12 June 2021 Exemplars
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupMEMORANDUM
QUESTION 1
1.1 D √√ (2)
1.2 D √√ (2)
1.3 A √√ (2)
1.4 C √√ (2)
1.5 C √√ (2)
1.6 D √√ (2)
1.7 B √√ (2)
1.8 B √√ (2)
1.9 B √√ (2)
1.10 C √√ (2) [20]
QUESTION 2
2.1
2.1.1 Homologous series √ (1)
2.1.2 Unsaturated. √
Contains a triple bond √/multiple bonds (2)
2.1.3 C5H9 √ (1)
2.1.4 5- ethlyl √ -2-methyl √ hex-3-yne √ OR 5-ethlyl-2-methyl-3-hexyne
Marking Criteria
- Hexyne √
- Side chains (ethyl and methyl) √
- Whole name correct √ (3)
2.2.1 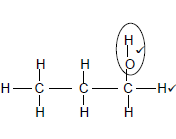
Marking criteria √
- Functional group correct (1/2)
- Whole structure correct (2/2) (2)
2.2.2 (Mild) heat √ in a water bath √ (2)
2.2.3 Esterification/ Condensation √ (1)
2.2.4 H2SO4 √ (1)
2.2.5 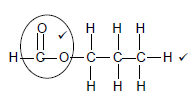
Marking criteria √
- Functional group √ correct (1/2)
- Whole structure correct (2/2) (2)
2.3
2.3.1 Compounds with the same molecular formula. √ but different structural formula. √ (2)
2.3.2 The C atom bonded to Br is bonded to one other C atom √√ OR The C atom bonded to Br is bonded to two hydrogen atoms (2)
2.3.3 1-bromo-2-methylpropane √√√
Marking Criteria
- Propane √
- (bromo and methyl) √
- Whole name correct √ (3)
[22]
QUESTION 3
3.1 The temperature at which the vapour pressure of a liquid equals the atmospheric pressure. √√ (2)
3.2 A √ Compound A has the lowest boiling point. √ (2)
3.3.1 Molar mass/ Molecular size/ Surface area √ (1)
3.3.2 Hydrogen bond √ (1)
3.4
- The molar mass/Molecular size/ surface area of the compounds increases from top to bottom in the table. √
- The compounds have London forces and (Hydrogen bonds) √
- The strength of London forces increases with an increase in molar mass/molecular size/surface area. √
- More energy is needed to overcome the intermolecular forces √ (4)
3.5.1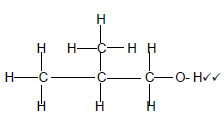
Marking criteria
- 3 Carbon atoms in longest chain with correct functional group (2)
3.5.2 B √ It has the same molecular formula √ (2)
3.5.3 Lower than √ (1)
3.5.4
- 2-methylpropan-1-ol has a smaller surface area than butan-1-ol. √
- Both compounds have London forces and (hydrogen bonds) √
- 2-methylpropan-1-ol have weaker London forces than butan-1-ol √
- Less energy is needed to overcome the intermolecular forces in 2-methylpropan-1-ol √
OR
- Butan-1-ol has a larger surface area than 2-methylpropan-1-ol √
- Both compounds have London forces and (hydrogen bonds) √
- Butan-1-ol have stronger Londonforces than 2-methylpropan-1-ol √
- More energy is needed to overcome the intermolecular forces in butan-1-ol √ (3)
3.6
- Methanoic acid and propan-1-ol have both Hydrogen bond and (London forces) √
- Methanoic acid have two sites for hydrogen bonds while propan-1-ol have only one site for hydrogen bonds. √
- Methanoic acid has stronger intermolecular forces than propan-1-ol √
- More energy is needed to overcome the intermolecular forces in methanoic acid. √
OR
- Methanoic acid and propan-1-ol have both Hydrogen bond and (London forces) √
- Methanoic acid have two sites for hydrogen bonds while propan-1-ol have only one site for hydrogen bonds. √
- Propan-1-ol has weaker intermolecular forces than methanoic acid √
- Less energy is needed to overcome the intermolecular forces in propan-1-ol. √ (4)
[22]
QUESTION 4
4.1 Process of breaking down long chain hydrocarbons √/alkanes into smaller more useful chains √ (2)
4.2
4.2.1 C4H10 √√ (2)
4.2.2 HIGH TEMPERATURE √ OR HIGH PRESSURE (1)
4.3
4.3.1 Substitution √ (1)
4.3.2 Elimination √ (1)
4.4 Hydrogenation √ (1)
4.5
4.5.1 Br2/Bromine √ (1)
4.5.2 Pt/Ni/Pd/Platinum/Nickel/Palladium √ (1)
4.6 CH3CH2CH2CHBr √√+ KOH → CH3CH = CHCH3 √√ + KBr + H2O
Marking criteria
- Organic reactant √√
- Organic product √√
- ALL Inorganic reagents correct √ (KOH, KBr and H2O) (5)
[15]
QUESTION 5
5.1 Change in concentration (of reactant/products) per unit time √√
OR
Amount/Volume/Mass of reactant/product used/formed per unit time (2)
5.2 RELEASED √
ΔH < 0 √/Energy of products is less than the energy of reactants/
Reaction exothermic (2)
5.3
5.3.1 INCREASES √ (1)
5.3.2 NO EFFECT √ (1)
5.4
5.4.1 After 120 s √ (1)
5.4.2 Concentration (of HCℓ) √ Surface area (of Zinc) √ OR temperature (2)
5.5
5.5.1 Rate / Tempo = Δv/Δt= 300 -0√ /120 – 0 √= 2,50 cm3∙s-1 √ (3)
5.5.2 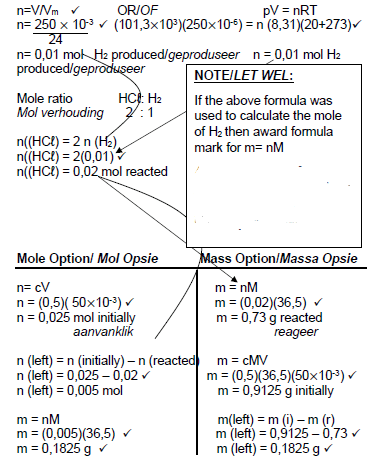 (7)
(7)
5.6
5.6.1 Catalyst √ OR Increases reaction rate (1)
5.6.2
- Catalyst lowers activation energy/provides an alternative path of lower activation energy √
- More particles have sufficient Ek OR more particles have Ek>Ea √
- More effective collisions per unit time/Frequency of effective collisions increase √√ (4)
[24]
QUESTION 6
6.1 Reaction in which products can be converted back to reactants √√ (2 or 0) (2)
6.2
6.2.1 Decreases √ (1)
6.2.2 Increases √(1)
6.3
- (When the temperature is decreased) the exothermic reaction is favoured √
- Forward reaction is favoured √ (2)
6.4 CALCULATION USING MOLES
Marking guideline
- Formula n=m/M
- Substitution of 28 g∙mol-1
- Use of ratio N2:H2:NH3
- Equilibrium amounts of N2 and H2 formed
- Division n equilibrium of N2, H2 and NH3 by 2
- Correct Kc expression
- Substitution into Kc expression
- Final answer
n = m/M √
= 7,84/28 √
= 0,28 mol
N2 + 3 H2 ⇋ 2 NH3
ni (mol) 0,28 0,6 0
Δn (mol) 0,06 0,18 0,12 Ratio √
ne (mol) 0,22 0,42 √ 0,12
ce 0,22/2 0,42/2 0,12/2 Division by 2 √
(mol∙dm-3) =0,11 0,21 0,06
Kc = [NH3]2/[N2].[H2]3 √
= 0,062/(0,11 x 0,213) √
= 3,53 √
CALCULATION USING CONCENTRATION
Marking guidelines
- Formula c=m/MV or c=n/v
- Substitution into c=m/MV or c=n/V
- Dividing by 2 for all concentration
- Use of ratio N2:H2:NH3
- Equilibrium concentrations of N2, H2 and NH3
- Correct Kc expression
- Substitution into Kc expression
- Final answer
- (Any one)
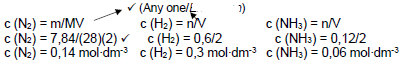
(dividing by) √
N2 + 3 H2 → 2 NH3 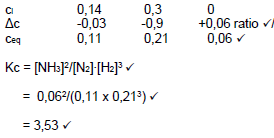 (8)
(8)
6.5 As pressure increases percentage yield increases. √√ (2)
6.6 MOLE OPTION
| Ratio N2 : H2 | Ratio N2 : H2 |
| 1 : 3 | 1 : 3 |
| n(H2) = 3 (0,28) √ n(H2) = 0,84 mol of H2 needed only 0,6 mol of H2 is available | n(N2) = 1/3 (0,6) n(N2) = 0,2 mol of N2 needed 0,28 mol of N2 is available |
0,84 mol van H2 word benodig 0,2 mol N2 word benodig
∴ H2 is the limiting reagent √ ∴ H2 is the limiting reagent
Theoretical yield/Teoretiese opbrengs = 0,6 x 2/3 = 0,4 mol √
% Yield = actual yield /theoretical yield × 100 %
= 0,12/0,4 x 100 √
= 30%
Pressure = 100 (atmospheres) √
CONCENTRATION OPTION
- V is constant
- Concentration ratio N2 : H2 Concentration ratio N2 : H2
1: 3 1 : 3
c(H2) = 3 (0,14) √ c(N2) = 1/3 (0,3)
c(H2) = 0,42 mol∙dm-3 of H2 required c(N2) = 0,1 mol∙dm-3
only 0,3 mol∙dm-3 is available 0,14 mol∙dm-3 is available
∴ H2 is the limiting reagent √
Theoretical yield = 0,3×2/3 √= 0,2 mol∙dm-3
- % yield = actual yield /theoretical yield ×100 %
- % yield = 0,06/0,2 ×100 % √
- % yield = 30 %
- Pressure 100 (atmosphere) √ (5)
[21]
QUESTION 7
7.1
7.1.1 Reaction of a salt with water √√ (2)
7.1.2 (Excess) Hydroxide/OH- ions are formed √
Hydroxide/ OH- is basic/alkaline √ (2)
7.1.3 H2O √ and HCO3- √ (2)
7.1.4 HCO3- can accept a proton (H+) (to form H2CO3) √
HCO3- can donate a proton (H+) (to form CO32-) √ (2)
7.2
7.2.1 Acid is a proton donor √√ (2)
7.2.2
- pH = - log [H3O+] √
1 = - log [H3O+] √
[H3O+] = 0,1 mol∙dm -3 √
[X] = 2 [H3O+]
[X] = 2(0,1)
[X] = 0,2 mol∙dm -3 √ (4)
7.2.3 It is a solution of known concentration √√ (2)
7.2.4 Bromothymol blue √ Acid X is a strong acid. KOH is a strong base. √
There is no hydrolysis of the salt produced, therefore the equivalence point of the titration would be within the range of indicator. √ (3)
7.2.5 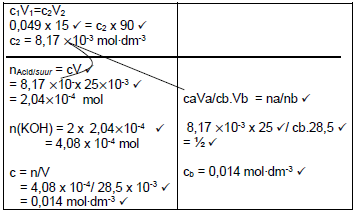 (7)
(7)
[26]
TOTAL: 150