PHYSICAL SCIENCES CHEMISTRY PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS JUNE 2016
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES (CHEMISTRY) P2
GRADE 12
JUNE 2016
NATIONAL SENIOR CERTIFICATE
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.This question paper consists of EIGHT questions.
- Answer QUESTION 5.8 on the attached GRAPH SHEET. Answer ALL the questions in theANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub questions, for example between QUESTION 2.1 and QUESTION 2.2.
- Write neatly and legibly.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- YOU ARE ADVISED TO USE THE ATTACHED DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four options are provided as possible answers to the following questions. Each question has only ONE correct answer. Write only the letter (A–D), corresponding to the correct answer of your choice, next to the question number (1.1–1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 A reaction in which products can be converted back to reactants is described as:
- Heterogeneous
- Homogeneous
- Reversible
- Spontaneous (2)
1.2 Which ONE of the following reaction conditions applies to esterification?
- Heat reaction mixture mildly over a water bath
- Apply mild heat directly to the reaction mixture
- Apply strong heat directly to the reaction mixture
- Add concentrated hydrochloric acid as a catalyst (2)
1.3 What NAME is given to the process of breaking down long chain hydrocarbons into more useful shorter chains?
- Hydrogenation
- Cracking
- Dehydrohalogenation
- Polymerisation (2)
1.4 Which ONE of the following changes will increase the rate of production of H2(g) in the reaction given below?
Mg(s) + H2SO4(aq) → MgSO4(aq) + H2(g)
- Increase in pressure by decreasing the volume
- Add water to the reaction mixture
- Increase the volume of the H2SO4(aq)
- Increase the concentration of the H2SO4(aq) (2)
1.5 Consider the reversible reaction: 3 Y2(g) ⇌ 2 Y3(g) ΔH = -80 kJ If the activation energy for the reverse reaction is 180 kJ, then the activation energy for the forward reaction is …
- -80 kJ.
- 80 kJ.
- 100 kJ.
- 180 kJ. (2)
1.6 An acid, HX has a concentration of 5 × 10-2 mol·dm-3 and Ka value equal to 103 at 25 ºC.
The solution of HX is most correctly described as a …
- dilute solution of a strong acid.
- dilute solution of a weak acid.
- concentrated solution of a weak acid.
- concentrated solution of a strong acid. (2)
1.7 To what volume must 20 cm3 of a 10 mol·dm-3 solution of potassium hydroxide (KOH) be diluted to obtain a 2 mol·dm-3 solution?
- 100 cm3
- 90 cm3
- 200 cm3
- 110 cm3 (2)
1.8 The sketch graph below represents changes in the volume of X2(g) as the following reaction proceeds in an open container.
2 XY(aq) + M → MY2(aq)+ X2(g)
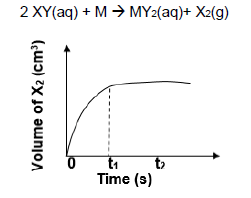
The horizontal section after time t1 means that the …
- reaction has stopped.
- reaction reaches equilibrium.
- rate of reaction increases.
- rate of reaction decreases. (2)
1.9 Consider the following reaction that is at equilibrium in a closed container
CoCℓ4(aq) + 6H2O(ℓ) ⇌ Co(H2O)62+ (aq) + 4Cℓ-(aq) ΔH < 0
What will be observed when a few drops of concentrated hydrochloric acid are added to the equilibrium mixture?
- The solution turns pink.
- The solution turns blue.
- The solution’s colour remains the same.
- The solution’s colour turns pink then turns blue. (2)
1.10 Distilled water ionises according to the following equation: 2 H2O(ℓ) ⇌ H3O+(aq) + OH-(aq)
The Kw values for distilled water are given below:
Kw = 1 × 10-14 at 25oC
Kw = 2,92 × 10-14 at 40 oC
Which statement is TRUE about distilled water as temperature increases from 25 oC to 40 oC?
- The water becomes acidic
- The water becomes alkaline
- [OH-] becomes higher than [H3O+]
- [OH-] remains equal to [H3O+] (2)
[20]
QUESTION 2
The letters A to F represent six organic compounds:
| A | Ethyl propanoate | B | Ethene |
| C | 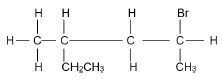 | D | 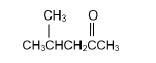 |
| E | Methanal | F | C4H10O |
2.1 Write down the …
2.1.1 general formula for the homologous series to which compound B belongs. (1)
2.1.2 name of the homologous series to which compound D belongs. (1)
2.1.3 letter of the compound that represents an aldehyde. (1)
2.2 Compound B undergoes polymerisation to form a polymer that is used to make plastic products.
2.2.1 Give a reason why compound B is classified as unsaturated. (2)
2.2.2 Classify the polymerisation as ADDITION or CONDENSATION. (1)
2.2.3 Write down the CONDENSED STRUCTURAL FORMULA of the polymer. (2)
2.3 Write down the …
2.3.1 IUPAC name of compound C. (3)
2.3.2 IUPAC name of compound D. (2)
2.4 Compound F is a secondary alcohol. Write down the …
2.4.1 STRUCTURAL FORMULA of compound F. (2)
2.4.2 IUPAC NAME of a CHAIN ISOMER of compound F. (2)
2.5 Compound A is prepared from the reaction between an alcohol and carboxylic acid in the presence of an inorganic acid.
Write down the …
2.5.1 IUPAC NAME of the carboxylic acid used. (2)
2.5.2 STRUCTURAL FORMULA of compound A. (2)
2.6 Compound B reacts with bromine (Br2).
2.6.1 Write down the MOLECULAR FORMULA of the product. (1)
2.6.2 Use a calculation to determine the percentage composition of the product. (5)
[27]
QUESTION 3
In the flow diagram below butan-1-ol is converted to its structural isomer, butan-2-ol.
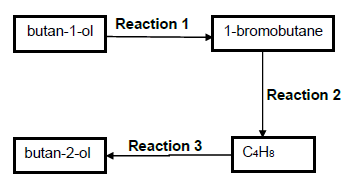
3.1 What type of structural isomers are butan-1-ol and butan-2-ol? (1)
3.2 For Reaction 1, write down the …
3.2.1 type of reaction of which this is an example. (1)
3.2.2 NAME or FORMULA of the INORGANIC reactant needed. (1)
3.3 For Reaction 2, write down …
3.3.1 the NAME or FORMULA of the INORGANIC reactant needed. (1)
3.3.2 ONE reaction condition. (1)
3.4 Write down the type of addition reaction of which Reaction 3 is an example. (1)
3.5 Butan-1-ol can be converted directly to the ORGANIC PRODUCT C4H8 without forming 1-bromobutane. Write down the NAME or FORMULA of the substance that can be used for this direct conversion. (1)
3.6 Using MOLECULAR FORMUAE write down a balanced equation for the complete combustion of the compound C4H8. (3)
[10]
QUESTION 4
The relationship between strength of intermolecular forces and boiling point is investigated using five organic compounds that belong to different homologous series.
| COMPOUND | BOILING POINT (oC) | |
| A | Butane | -1 |
| B | Butan-2-one | 79,5 |
| C | Butan-1-ol | 117,4 |
| D | Butanoic acid | 163,5 |
| E | Pentanoic acid | 187 |
4.1 Which compound in the table is a gas at room temperature? (1)
4.2 Define the term homologous series. (2)
4.3 A type of van der Waals force exists between molecules of compound A and also between molecules of compounds B, C, D and E. Write down the NAME of the Van der Waals force. (1)
4.4 Refer to the TYPE and STRENGTH of intermolecular forces to explain the difference in the boiling points between:
4.4.1 Compounds A and B (3)
4.4.2 Compounds C and D (3)
4.5 Consider compounds D and E.
4.5.1 Which compound has a HIGHER vapour pressure? (1)
4.5.2 Refer to MOLECULAR STRUCTURE, TYPE and STRENGTH of intermolecular forces to explain the answer to QUESTION 4.5.1. (3)
[14]
QUESTION 5
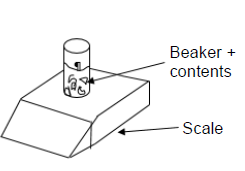
A certain mass of calcium carbonate chunks is added to EXCESS hydrochloric acid solution in an open beaker placed on a scale as shown below. The equation for the reaction is as follows:
CaCO3(s) + 2HCℓ(aq) → CaCℓ2(aq) + CO2(g) + H2O(ℓ)
The initial temperature of the reaction flask is 30 oC. The data in the table was obtained for the reaction.
| Time (minutes) | Mass of beaker and contents (g) |
| 0 | 192,4 |
| 1 | 188,8 |
| 2 | 188,0 |
| 3 | 187,4 |
| 4 | 187,1 |
| 5 | 186,7 |
| 6 | 186,7 |
5.1 Is the reaction mixture HETEROGENEOUS or HOMOGENEOUS? (1)
5.2 Give a reason why the mass of the contents of the beaker decreases as the reaction proceeds. (1)
5.3 How long (in minutes) did the reaction take to reach completion? (1)
5.4 Calculate the average rate of reaction during the interval 0 to 1 minute in grams per minute. (3)
5.5 The rate of reaction decreases as the reaction proceeds. Give TWO reasons why the reaction rate decreases. (2)
5.6 Apart from CO2, write the NAME or FORMULA of another substance that is not present in the container after 6 minutes. (1)
5.7 Calculate the mass of calcium carbonate consumed after completion of the reaction. (5)
5.8 Plot a graph of mass of contents of beaker versus time for the time interval from the 0th to the 6th minute. (A graph paper is provided at the back). (4)
NOTE: The graph is not a straight line.
(ATTACH THIS GRAPH SHEET TO THE ANSWERBOOK.)
5.9 Use the collision theory to explain how the rate of the above reaction will change when the initial temperature is changed to 50 oC. (4)
[22]
QUESTION 6
The following reaction reaches chemical equilibrium in a closed container at 1000 oC.
2AX3(g) ⇌ 2AX2(g) + X2(g)
The course of reaction is illustrated in the graph below:
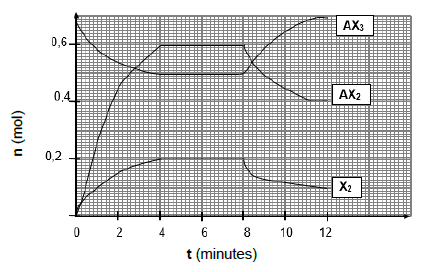
6.1 Explain the meaning of the term chemical equilibrium. (2)
6.2 Use the graph to determine the …
6.2.1 time the reaction took to reach chemical equilibrium for the first time. (1)
6.2.2 number of moles of AX3 at the first equilibrium. (1)
6.3 Calculate the volume of the container if Kc = 2,5 × 10-2 at 1000 oC. (6)
6.4 Is the yield, HIGH or LOW at 1000 oC? Give a reason. (2)
6.5 The change in the number of moles at t = 8 minutes is caused by a DECREASE in temperature.
Is the forward reaction ENDOTHERMIC or EXOTHERMIC? Explain your answer by using Le Chatelier’s principle. (4)
6.6 What effect will the addition of a suitable catalyst have on the value of Kc? Write down only DECREASES, INCREASES or REMAINS THE SAME. (1)
[17]
QUESTION 7
7.1 Oxalic acid, (COOH)2 ,ionises in two steps as shown below.
(COOH)2(s) + H2O(ℓ) ⇌ H(COO)2 -(aq) + H3O+(aq) Ka = 5,4 × 10-2 at 25oC
H(COO)2 -(aq) + H2O(ℓ) ⇌ (COO)2 2-(aq) + H3O+(aq) Ka = 5,4 × 10-5 at 25oC
7.1.1 Write down in words what the symbol, Ka, stands for. (1)
7.1.2 Why is the temperature at which the Ka is calculated always given? (1)
7.1.3 H2O is acting as a base in both reactions. Write down the FORMULA of a substance that acts as an ampholyte in the reactions. (1)
7.1.4 Write down the net equation for the ionisation of oxalic acid. (3)
7.2 A sodium hydroxide (NaOH) solution of volume 40 cm3 and concentration 1 mol·dm-3 is prepared.
7.2.1 Calculate the mass of sodium hydroxide needed to prepare the solution. (4)
The 40 cm3 of sodium hydroxide solution of concentration 1 mol·dm-3 is added to 50 cm3 of a 0,06 mol·dm-3 sulphuric acid (H2SO4) solution in a flask. The reaction taking place in the flask is given below:
2NaOH(aq) + H2SO4(aq) → Na2SO4(aq) + 2H2O(ℓ)
Calculate the …
7.2.2 initial number of moles of sulphuric acid in the flask. (3)
7.2.3 pH of the solution in the flask after the completion of the reaction. (8)
7.3 A titration between solutions of a strong base and standard ethanoic acid (CH3COOH) solution is performed. The acid is added from apparatus X into a flask under which a white tile is placed until a point where the indicator changes colour is reached.
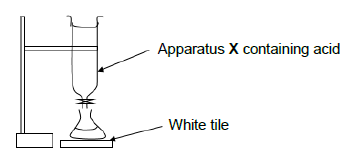
7.3.1 Write down a term for the underlined phrase. (1)
7.3.2 Name apparatus X from which the acid is added. (1)
7.3.3 What is the purpose of the white tile? (1)
7.3.4 A learner performing the titration accidentally adds three drops of the acid after the indicator has changed colour. When she measures the pH of the solution after adding the three drops she finds out that the solution has a pH > 7. With the aid of a balanced equation, explain why the solution has a pH > 7. (4)
[28]
QUESTION 8
Three reactions that lead to the formation of nitric acid (HNO3) are shown below:
Pt
Reaction 1: 4NH3(g) + 5O2(g) → 4NO(g) + 6H2O(g)
Reaction 2: 2NO(g) + O2(g) ⇌ 2NO2(g) ΔH = -149,1 kJ
Reaction 3: 4NO2(g) + O2(g) + H2O → 4HNO3
8.1 In Reaction 1, platinum (Pt) acts as a catalyst.
What NAME is given to the energy that a catalyst changes in a chemical reaction? (1)
8.2 Reaction 2 reaches equilibrium in a closed container.
8.2.1 Is the reaction EXOTHERMIC or ENDOTHERMIC? Give a reason. (2)
8.2.2 Write down TWO changes that must be made to increase the YIELD of NO2. (2)
8.2.3 What is the value of ΔH per mole of NO2 formed? (1)
8.3 Nitric acid reacts with ammonia (NH3) to produce ammonium nitrate (NH4NO3).
8.3.1 Write down the NAME of the type of reaction between an acid and a base. (1)
8.3.2 Which particle (PROTON or ELECTRON) is transferred during the reaction mentioned in QUESTION 8.3.1? (1)
8.3.3 To determine the percentage purity of an IMPURE ammonium nitrate sample, the sample is dissolved in water and allowed to react with a solution of sodium hydroxide according to the balanced equation:
NH4NO3(aq) + NaOH(aq) → NaNO3(aq) + NH3(g) + H2O(ℓ)
0,204 g of the IMPURE sample of ammonium nitrate (NH4NO3) neutralises exactly 2,4 × 10-3 mol of sodium hydroxide (NaOH). Calculate the percentage purity of the ammonium nitrate sample. (4)
[12]
TOTAL: 150
NATIONAL SENIOR CERTIFICATE
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 × 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | 273 K |
| Charge on electron | e | e -1,6 × 10-19 C |
| Avogadro’s constant | NA | 6,02 × 1023 mol-1 |
TABLE 2: FORMULAE
n = m or M | c= n or V c = m MV 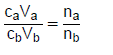 | pH= -log[H3O+] Kw = [H3O+][OH-] = 1x10-14 at 298K |
| Eθ cell = Eθ cathode–Eθ anode Eθ cell = Eθ reduction–Eθ oxidation Eθ cell = Eθ oxidising agent–Eθ reducing agent | ||
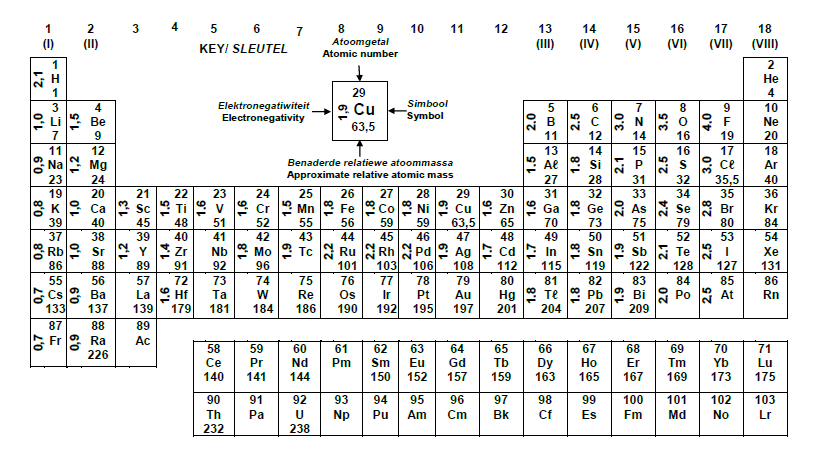
TABLE 3: THE PERIODIC TABLE OF ELEMENTS
GRAPH SHEET FOR QUESTION 5.8
NAME OF LEARNER: ..................................................................... DATE: ......................
NAME OF SCHOOL: .......................................................... GRADE 12: ............
GRAPH OF MASS OF BEAKER + CONTENTS vs. TIME