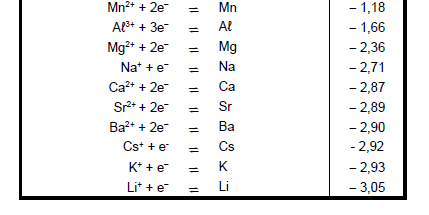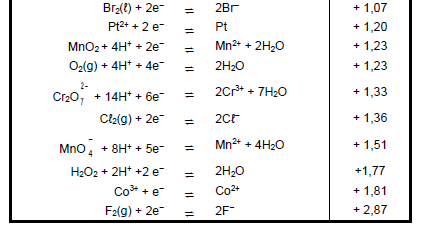PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS MAY/JUNE 2019
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY (PAPER 2)
GRADE 12
NATIONAL SENIOR CERTIFICATE EXAMINATIONS
MAY/JUNE 2019
INSTRUCTIONS AND INFORMATION
- Write your examination number and centre number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 D.
1.1 Which ONE of the following is a SECONDARY alcohol?
- Ethanol
- Butan-1-ol
- Butan-2-ol
- 2-methylbutan-1-ol (2)
1.2 Which ONE of the following will RAPIDLY decolourise bromine water?
- CH3CHCH2
- CH3CH2CH3
- CH3COOCH3
- CH3CH2COOH (2)
1.3 A FUNCTIONAL ISOMER of ethyl propanoate is …
- C4H9CHO.
- C5H11OH.
- C4H9COOH.
- CH3(CH2)3CHO. (2)
1.4 Consider the balanced equation for a chemical reaction below.
2NO(g) + O2(g) → 2NO2(g)
The activation energy of the forward and reverse reactions are 156 kJ·mol-1 and 175 kJ·mol-1 respectively.
The heat of reaction, in kJ·mol-1, for this reaction is …
- –19.
- +19.
- +331.
- –331. (2)
1.5 The reaction given below reaches equilibrium in a closed container. The Kc value is 0,04 at a certain temperature.
2SO2(g) + O2(g) ⇌ 2SO3(g) ΔH < 0
Which ONE of the following factors will change the Kc value to 0,4?
- Increase in pressure
- Decrease in pressure
- Increase in temperature
- Decrease in temperature
1.6 Which ONE of the following statements best describes a state of dynamic equilibrium?
- The limiting reagent has been used up.
- The forward and reverse reactions have stopped.
- The rates of the forward and reverse reactions are equal.
- The concentration of products equals the concentration of reactants. (2)
1.7 During a titration to determine the concentration of an acid using a standard base, a learner pipettes the base into a conical flask. She then uses a small amount of water to rinse the inside of the flask so that all the base is part of the solution in the flask.
How will the extra water added to the flask affect the results of this titration?
The concentration of the acid …
- cannot be determined.
- will be lower than expected.
- will be higher than expected.
- will be the same as expected. (2)
1.8 The standard reduction potentials for two substances used to set up a galvanic cell are as follows:
Sn2+ + 2e– ⇌ Sn Eθ = – 0,14 V
Cu2+ + 2e– ⇌ Cu Eθ = 0,34 V
Which ONE of the following combinations gives the substances formed at each electrode when the cell is functioning?
| Cathode | Anode | |
| A | Cu2+ | Sn |
| B | Sn | Cu2+ |
| C | Sn2+ | Cu |
| D | Cu | Sn2+ |
(2)
1.9 Which ONE of the following half-reactions takes place at the POSITIVE ELECTRODE of an electrochemical cell used to electroplate an iron rod with silver?
- Ag+ + e– → Ag
- Fe2+ + 2e– → Fe
- Ag → Ag+ + e–
- Fe → Fe2+ + 2e– (2)
1.10 Which ONE of the following elements is a primary nutrient?
- Potassium
- Sulphur
- Oxygen
- Carbon (2)
[20]
QUESTION 2 (Start on a new page.)
The letters A to F in the table below represent six organic compounds.
| A |  | B |  |
| C | CH3CCCH2CH3 | D | Butyl propanoate |
| E |  | F |  |
2.1 Is compound C SATURATED or UNSATURATED? Give a reason for the answer. (2)
2.2 Write down the LETTER that represents each of the following:
2.2.1 An ester (1)
2.2.2 A FUNCTIONAL ISOMER of butanal (1)
2.2.3 A compound with the general formula CnH2n-2 (1)
2.2.4 A compound used as reactant in the preparation of compound D (1)
2.3 Write down the STRUCTURAL FORMULA of:
2.3.1 The functional group of compound C (1)
2.3.2 Compound D (2)
2.3.3 A CHAIN ISOMER of compound A (2)
2.4 Write down the:
2.4.1 IUPAC name of compound F (3)
2.4.2 Balanced equation, using MOLECULAR FORMULAE, for the complete combustion of compound A (3)
[17]
QUESTION 3 (Start on a new page.)
Three compounds are used to investigate one of the factors that influences boiling point. The results obtained are shown in the table below.
| COMPOUND | MOLECULAR MASS (g∙mol-1) | BOILING POINT (°C) | |
| A | Butane | 58 | -0.5 |
| B | Propan-1-ol | 60 | 98 |
| C | Ethanoic acid | 60 | 118 |
3.1 In one investigation the boiling points of compound B and compound C are compared.
3.1.1 Is this a fair investigation? Write down YES or NO. Refer to the data in the table and give a reason for the answer. (2)
3.1.2 Write down the independent variable for this investigation. (1)
3.2 Which ONE of the compounds (A, B or C) has the highest vapour pressure? Give a reason for the answer. (2)
3.3 Refer to the intermolecular forces present in each compound and FULLY explain the trend in boiling points, as shown in the above table. (5)
3.4 Which compound, BUTAN-1-OL or PROPAN-1-OL, has the higher boiling point? Give a reason for the answer. (2)
[12]
QUESTION 4 (Start on a new page.)
4.1 The balanced equation for a polymerisation reaction is shown below.
Write down the:
4.1.1 Type of polymerisation reaction represented by the equation (1)
4.1.2 IUPAC name of the monomer (1)
4.1.3 IUPAC name of the polymer (1)
4.2 Propan-1-ol undergoes two different reactions, as shown in the diagram below.
Write down the:
4.2.1 Type of reaction represented by reaction 2 (1)
4.2.2 Function of concentrated H2SO4 in reaction 2 (1)
4.2.3 IUPAC name of compound X (2)
4.2.4 STRUCTURAL FORMULA of compound Y (2)
4.2.5 Type of reaction represented by reaction 3 (1)
4.2.6 IUPAC name of compound Z (2)
[12]
QUESTION 5 (Start on a new page.)
Learners use the reaction of a sodium thiosulphate solution with dilute hydrochloric acid to investigate several factors that affect the rate of a chemical reaction. The balanced equation for the reaction is:
Na2S2O3(aq) + 2HCℓ(aq) → 2NaCℓ(aq) + SO2(g) + S(s) + H2O(ℓ)
5.1 Define reaction rate. (2)
Three investigations (I, II and III) are carried out.
5.2 INVESTIGATION I
The results obtained in INVESTIGATION I are shown in the graph below.
For this investigation, write down the:
5.2.1 Dependent variable (1)
5.2.2 Conclusion that can be drawn from the results (2)
5.3.1 What does line P represent? (1)
5.3.2 Which curve (A or B) was obtained at the higher temperature? (1)
5.3.3 Explain, in terms of the collision theory, how an increase in temperature influences the rate of a reaction. (4)
5.3 INVESTIGATION II
The Maxwell-Boltzmann distribution curves, A and B, below represent the number of particles against kinetic energy for the reaction at two different temperatures.
5.4 INVESTIGATION III
The potential energy diagrams, X and Y, below represent the reaction under two different conditions.
Give a reason why curve Y differs from curve X. (1)
5.5 In one of the investigations, 100 cm3 of 0,2 mol·dm–3 HCℓ(aq) reacts with excess Na2S2O3(aq) and the solution is then filtered. After filtration of the solution, 0,18 g of sulphur is obtained. Calculate the PERCENTAGE YIELD of sulphur. (6)
[18]
QUESTION 6 (Start on a new page.)
6.1 Write down the meaning of the double arrow used in the equation above. (1)
6.2 Give ONE reason why ammonia is removed from the reaction vessel as quickly as it forms. (1)
The graph below shows the percentage yield of ammonia at different temperatures and pressures.
6.3 Write down the percentage yield of ammonia at a temperature of 450 °C and a pressure of 200 atmospheres. (1)
The balanced equation below represents the reaction used in the Haber process to produce ammonia.
N2(g) + 3H2(g) ⇌ 2NH3(g) ΔH < 0
In industry the product is removed as quickly as it forms.
6.4 Refer to Le Chatelier's principle to explain EACH of the following deductions made from the graph:
6.4.1 For a given pressure, the yield of ammonia at 500 °C is much lower than that at 350 °C (3)
6.4.2 For a given temperature, the yield of ammonia at 350 atmospheres is much higher than that at 150 atmospheres (2)
6.5 A technician prepares NH3(g) by reacting 6 moles of H2(g) and 6 moles of N2(g).
6.5.1 Calculate the maximum number of moles of NH3(g) that can be obtained in this reaction. (2)
6.5.2 The above reaction now takes place in a 500 cm3 container at a temperature of 350 °C and a pressure of 150 atmospheres. The system is allowed to reach equilibrium.
Use the graph above and calculate the equilibrium constant, Kc, for this reaction under these conditions. (7)
[17]
QUESTION 7 (Start on a new page.)
7.1 Define a base in terms of the Arrhenius theory. (2)
7.2 Explain how a weak base differs from a strong base. (2)
7.3 Write down the balanced equation for the hydrolysis of NaHCO3. (3)
7.4 A learner wishes to identify element X in the hydrogen carbonate, XHCO3. To do this she dissolves 0,4 g of XHCO3 in 100 cm3 of water. She then titrates all of this solution with a 0,2 mol dm-3 hydrochloric acid (HCℓ) solution. Methyl orange is used as the indicator during the titration.
7.4.1 Calculate the pH of the hydrochloric acid solution. (3)
7.4.2 Give a reason why methyl orange is a suitable indicator in this titration. (1)
At the endpoint she finds that 20 cm3 of the acid neutralised ALL the hydrogen carbonate solution. The balanced equation for the reaction is:
XHCO3(aq) + HCℓ(aq) → XCℓ(aq) + CO2(g) + H2O(ℓ)
7.4.3 Identify element X by means of a calculation. (6)
[17]
QUESTION 8 (Start on a new page.)
The electrochemical cell below functions under standard conditions.
8.1 Give a reason why platinum is used as the electrode in half-cell A. (1)
8.2 Write down the:
8.2.1 Energy conversion that takes place in this cell (1)
8.2.2 Half-reaction that takes place at the cathode (2)
8.2.3 Cell notation for this cell (3)
8.3 Calculate the initial emf of this cell. (4)
8.4 Silver chloride is an insoluble salt.
What will be the effect on the cell potential when a small amount of silver nitrate solution, AgNO3(aq), is added to half-cell A? Choose from INCREASES, DECREASES or REMAINS THE SAME. (2)
[13]
QUESTION 9 (Start on a new page.)
The diagrams below represent two electrochemical cells.
P, Q, X and Y are carbon electrodes.
When cell B is functioning, the mass of electrode X increases.
9.1 What type of electrochemical cell, GALVANIC or ELECTROLYTIC, is illustrated above? (1)
9.2 Write down the half-reaction that takes place at electrode Q. (2)
9.3 The products formed in the two cells are compared.
9.3.1 Name ONE substance that is produced in BOTH cells. (1)
9.3.2 Write down the LETTERS of the TWO electrodes where this product is formed. Choose from P, Q, X and Y. (2)
9.4 Is electrode X the CATHODE or the ANODE? Give a reason for the answer. (2)
9.5 Write down the net (overall) cell reaction that takes place in cell B. (3)
[11]
QUESTION 10 (Start on a new page.)
10.1 The four steps in the manufacture of an inorganic fertiliser are listed below. These steps are NOT written in the order in which they occur.
Step I: Sulphuric acid reacts with ammonia to produce ammonium sulphate.
Step II: Sulphur dioxide reacts with oxygen to produce sulphur trioxide.
Step III: Oleum is diluted with water to produce sulphuric acid.
Step IV: Sulphur trioxide is bubbled in concentrated sulphuric acid to produce oleum.
Write down the:
10.1.1 Correct order in which the steps occur in the preparation of the inorganic fertiliser by using the numbers I to IV (1)
10.1.2 Balanced chemical equation for step I (3)
10.1.3 NAME of the catalyst used in step II (1)
10.1.4 Balanced chemical equation for step IV (3)
10.1.5 Reason why sulphur trioxide is NOT dissolved in water in step IV (1)
10.2 The diagram below shows a bag of NPK fertiliser. One of the numbers of the NPK ratio on the bag is labelled as X.
If the mass of potassium in the bag is 3,33 kg, calculate the value of X. (4)
[13]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | e -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m | n = N NA |
| c = n V or c = m MV | n = V Vm |
| caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | |
| Eθ cell = Eθ cathode – Eθ anode Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |
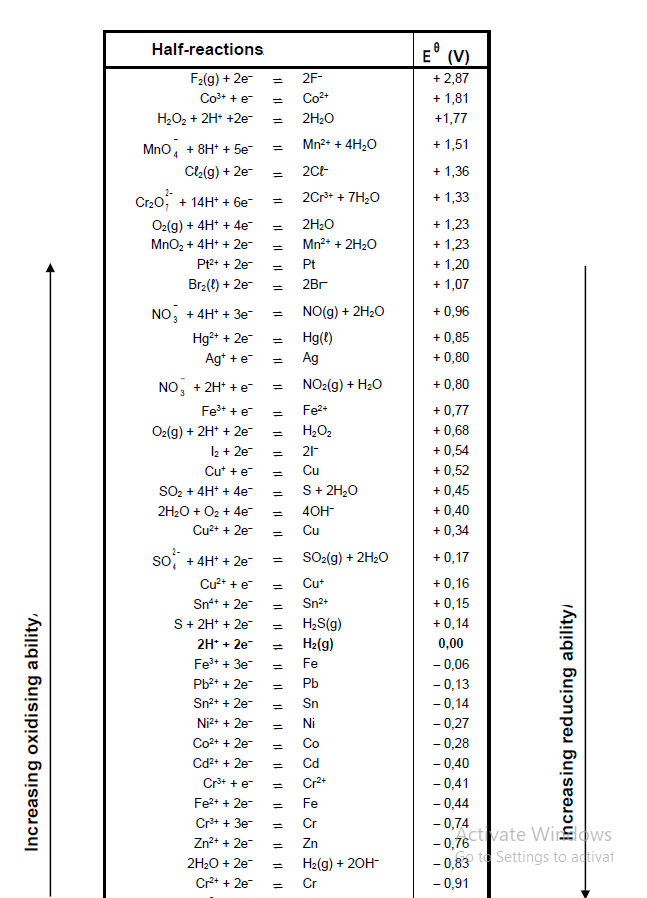
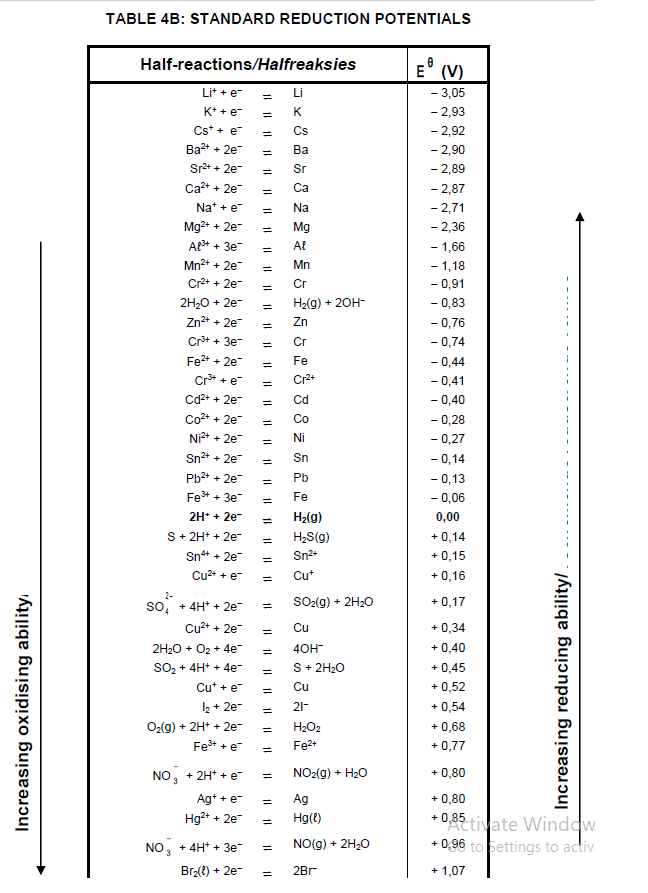
TABLE 4A: STANDARD REDUCTION POTENTIALS