Technical Sciences Paper 2 Memorandum - Grade 12 June 2021 Exemplars
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupMEMORANDUM
QUESTION 1
1.1 D √√ (2)
1.2 C √√ (2)
1.3 B √√ (2)
1.4 A √√ (2)
1.5 B √√ (2) [10]
QUESTION 2
2.1 Combustion √ (1)
2.2 Structural isomers √ (1)
2.3 Polymers √ (1)
2.4 n-type (semiconductor) √ (1) [4]
QUESTION 3
3.1
3.1.1 An organic molecule is a molecule which contains carbon atoms. √√(2)
3.1.2 CnH2n √ (1)
3.1.3 Alcohols √ (1)
3.1.4 Hydroxyl group √ (1)
3.1.5 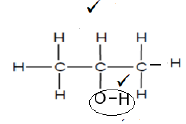 (2)
(2)
3.2
3.2.1 Esters: Dipole-dipole intermolecular forces √ (1)
Alcohols: Hydrogen bond √ (1)
3.2.2 Methanoic acid √ (1)
3.2.3 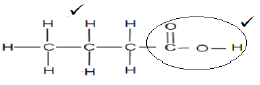 (2)
(2)
3.2.4 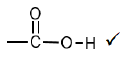 (1)
(1)
3.3
3.3.1 Same molecular formula, C5H12 (1)
3.3.2 Different chain length (1)
3.3.3 Alkanes (1)
3.4
3.4.1 Pentane (1)
3.4.2 2-methyl butane (2)
3.4.3 Pentane (1)
3.4.4 B (1)
3.4.5 B is branched; the more branches in the organic compound, the smaller the surface area. (2)
3.5
3.5.1 Plastics are synthetic materials derived from organic compounds.
3.5.2
USE | |
B | Food-packaging plastics |
C | Squeezable bottles |
E | Flexible water pipes |
(Any 2 x 1)(2) [27]
QUESTION 4
4.1 Vapour pressure is the pressure exerted by a vapour at equilibrium with its liquid in a closed system. √√ (2)
4.2 4.2.1 Dipole-dipole intermolecular forces √ (1)
4.2.2 Dipole-dipole intermolecular forces √ (1)
4.3 Viscosity of chlorohexane is HIGHER. √
Chlorohexane has stronger dipole-dipole intermolecular forces.
Pentane has weaker dispersion / London forces. √ (2)
4.4 Pentane √ - It has weak London forces. √ Less energy will be required to overcome intermolecular forces. √ (3)
4.5 London forces √√ (2)
[11]
QUESTION 5
5.1 Hydrocarbons are organic compounds that consist only of hydrogen and carbon atoms. √ (1)
5.2
5.2.1 CH4 + 2O2 √ CO2 + 2H2O √ √ bal (3)
5.2.2 Excess oxygen √ (1)
[5]
QUESTION 6
6.1 6.1.1 Hydrolysis √ (1)
6.1.2 Hydration √ (1)
6.1.3 Esterification √ (1)
6.2
6.2.1 Hexan-2-ol or 2-hexanol √ (1)
6.2.2 1-bromohexane √ (1)
6.2.3 Water √ (1)
6.3
6.3.1 Pt/Pd/Ni as a catalyst √ (1)
6.3.2 No water added, unreactive solvent, CHCl2, CCl4 √ (1)
6.4 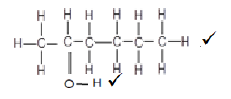 (2)
(2)
6.5
6.5.1 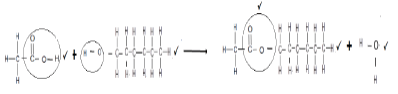 (4)
(4)
6.5.2 Ethyl hexanoate √ (1)
6.5.3 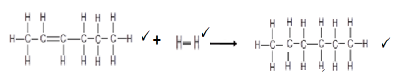 (3)
(3)
[18]
TOTAL: 75