PHYSICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS NOVEMBER 2021
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of NINE questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- You are advised to use the attached DATA SHEETS.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, e.g. 1.11 E.
1.1 Which formula shows the way in which atoms are bonded in a molecule but does not show all the bond lines?
- Empirical
- Molecular
- Structural
- Condensed structural
(2)
1.2 Which ONE of the following compounds has hydrogen bonds between its molecules?
- CH3(CH2)2CH3
- CH3COCH2CH3
- CH3COOCH2CH3
- CH3CH(OH)CH2CH3
(2)
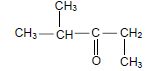
1.3 Consider the compound below.
Which ONE of the following is the IUPAC name of this compound?
- 2-methylpentan-3-one
- 4-methylpentan-3-one
- 2,3-dimethylbutan-2-one
- 2,2,4-trimethylpropan-2-one
(2)
1.4 A 2 g piece of magnesium reacts with EXCESS hydrochloric acid according to the following balanced equation:
Mg(s) + 2HCℓ(aq) → MgCℓ2(aq) + H2(g)
Which ONE of the following changes will INCREASE the YIELD of H2(g)?
- Crush the piece of magnesium.
- Use a 3 g piece of magnesium.
- Use a greater volume of the acid.
- Use a higher concentration of the acid.
(2)
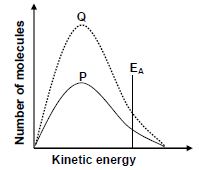
1.5 The Maxwell-Boltzmann distribution curve P represents the number of molecules against kinetic energy for a certain reaction.
Curve Q is obtained after a change was made to one reaction condition.
Which ONE of the following changes resulted in curve Q?
- Addition of a catalyst
- Increase in temperature
- Increase in activation energy
- Increase in the concentration of the reactants
(2)
1.6 The expression for the equilibrium constant (Kc) of a hypothetical reaction is given as follows:
Kc = [X]³
[Y]² [Z]
Which ONE of the following equations for a reaction at equilibrium matches
the above expression?
- Z(g) + 2Y(g) ⇌ 3X(s)
- Z(aq) + 2Y(aq) ⇌ 3X(ℓ)
- Z(g) + Y2(g) ⇌ 3X(aq) + Q(s)
- Z(aq) + 2Y(aq) ⇌ 3X(aq) + Q(s)
(2)
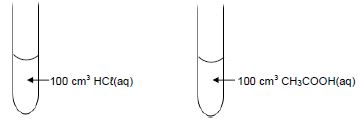
1.7 Two dilute acids of equal concentrations are added to separate test tubes as shown below.
Consider the following statements regarding these acids:
- The pH of each is less than 7.
- Both will react at the same rate with 5 g of magnesium powder.
- Both will neutralise the same number of moles of NaOH(aq).
Which of the statements above is/are TRUE?- I only
- I, II and III
- I and III only
- II and III only (2)
1.8 Which ONE of the following is the conjugate base of H2PO-4 ?
- PO3-4
- HPO2-4
- H3PO4
- H4PO4
(2)
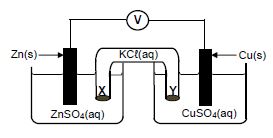
1.9 The diagram below represents a voltaic cell.
Which ONE of the following correctly describes the movement of ions in the cell?
| TYPE OF IONS | DIRECTION OF MOVEMENT | |
| A | Cℓ─(aq) | Y to X |
| B | SO2-4 (aq) | X to Y |
| C | Cu2+(aq) | Y to X |
| D | K+(aq) | Y to X |
(2)
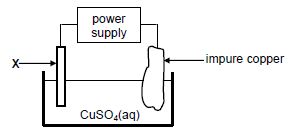
1.10 The diagram below represents a cell that is used for the refining of copper.
Which ONE of the following statements is TRUE?
- X is made of platinum.
- The mass of X increases.
- X is the electrode where oxidation takes place.
- X is connected to the positive terminal of the power supply. (2)
[20]
QUESTION 2 (Start on a new page.)
The letters A to H in the table below represent eight organic compounds.
| A | 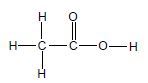 | B | 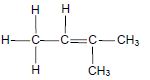 |
| C | CH3CH2CH2COCH3 | D | C2H6O |
| E | C2H4 | F | 3-methylbutan-2-one |
| G | 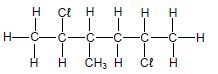 | H | 3-methylbutanal |
2.1 Define the term unsaturated compound. (2)
2.2 Write down the:
2.2.1 Letter that represents an UNSATURATED compound (1)
2.2.2 NAME of the functional group of compound C (1)
2.2.3 Letter that represents a CHAIN ISOMER of compound C (2)
2.2.4 IUPAC name of compound G (3)
2.2.5 General formula of the homologous series to which compound E belongs (1)
2.3 Define the term functional isomers. (2)
2.4 For compound A, write down the:
2.4.1 Homologous series to which it belong (1)
2.4.2 STRUCTURAL FORMULA of its FUNCTIONAL isomer (2)
2.5 Compound D undergoes a dehydration reaction. Write down the:
2.5.1 IUPAC name of compound D (1)
2.5.2 Letter that represents a product of this reaction (1)
2.5.3 NAME or FORMULA of the inorganic reactant that is used in this reaction (1)
[18]
QUESTION 3 (Start on a new page.)
The melting points and boiling points of four straight-chain ALKANES are shown in the table below.
| COMPOUND | MELTING POINT (°C) | BOILING POINT (°C) |
| Pentane | -130 | 36,1 |
| Hexane | -94 | 69 |
| Heptane | -90,6 | 98,4 |
| Octane | -57 | 125 |
3.1 Define the term melting point. (2)
3.2 Write down the general conclusion that can be made about the melting points of straight-chain alkanes. (2)
3.3 Name the type of Van der Waals forces between molecules of octane. (1)
3.4 Write down the predominant phase of the following alkanes at -100 °C.
Choose from GAS, LIQUID or SOLID.
3.4.1 Pentane (1)
3.4.2 Octane (1)
3.5 Hexane is now compared to 2,2-dimethylbutane.
3.5.1 Is the molecular mass of hexane GREATER THAN, LESS THAN or EQUAL to that of 2,2-dimethylbutane?
Give a reason for the answer. (2)
3.5.2 Is the boiling point of 2,2-dimethylbutane HIGHER THAN, LOWER THAN or EQUAL TO that of hexane? (1)
3.5.3 Fully explain the answer to QUESTION 3.5.2. (3)
[13]
QUESTION 4 (Start on a new page.)
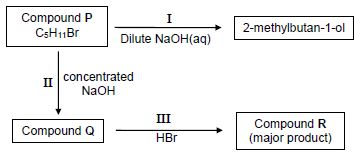
4.1 Compound P is used as a starting reactant in each of two reactions as shown in the flow diagram below.
I, II and III represent organic reactions.
4.1.1 Name the type of reaction represented by I. (1)
4.1.2 Is 2-methylbutan-1-ol a PRIMARY, SECONDARY or TERTIARY alcohol?
Give a reason for the answer. (2)
4.1.3 Write down the STRUCTURAL FORMULA of compound P. (3)
4.1.4 Name the type of reaction represented by II. (1)
4.1.5 To which homologous series does compound Q belong? (1)
4.1.6 Name the type of reaction represented by III.
Choose from ADDITION, ELIMINATION or SUBSTITUTION. (1)
4.1.7 Write down the IUPAC name of compound R. (2)
4.2 1,2-dibromopropane can be prepared from but-2-ene by a three-step process as shown in the flow diagram below.
4.2.1 Using CONDENSED STRUCTURAL FORMULAE, write down a balanced equation for step 1. Indicate the reaction conditions on the arrow. (4)
4.2.2 Write down the type of reaction in step 2. (1)
4.2.3 Write down the IUPAC name of compound B. (2)
4.2.4 Using CONDENSED STRUCTURAL FORMULAE, write down a balanced equation for step 3. (3)
[21]
QUESTION 5 (Start on a new page.)
The reaction of 15 g of an IMPURE sample of calcium carbonate, CaCO3, with EXCESS hydrochloric acid, HCℓ, of concentration 1,0 mol∙dm-3, is used to investigate the rate of a reaction. The balanced equation for the reaction is:
CaCO3(s) + 2HCℓ(aq) → CaCℓ2(aq) + H2O(ℓ) + CO2(g)
The volume of CO2(g) produced is measured at regular intervals. A sketch graph representing the total volume of carbon dioxide gas produced as a function of time is shown below.
5.1 Define the term reaction rate. (2)
5.2 Give a reason why the gradient of the graph decreases between t2 and t3. (1)
5.3 Changes in the graph between t1 and t2 are due to temperature changes within the reaction mixture.
5.3.1 Is the reaction EXOTHERMIC or ENDOTHERMIC? (1)
5.3.2 Explain the answer by referring to the graph. (3)
5.4 The percentage purity of the sample is 82,5%.
Calculate the value of X on the graph assuming that the gas is collected at 25 °C. Take the molar gas volume at 25 °C as 24 000 cm3. (5)
5.5 How will the reaction rate change if 15 g of a PURE sample of CaCO3 reacts with the same HCℓ solution?
Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
5.6 Use the collision theory to explain the answer to QUESTION 5.5. (2)
[15]
QUESTION 6 (Start on a new page.)
Consider the balanced equation below for a hypothetical reaction that takes place in a sealed 2 dm3 container at 300 K.
2P(g) + Q2(g) ⇌ 2PQ(g)
6.1 Define the term chemical equilibrium. (2)
6.2 The amount of each substance present in the equilibrium mixture at 300 K is shown in the table below.
| AMOUNT (mol) AT EQUILIBRIUM | |
| P | 0,8 |
| Q2 | 0,8 |
| PQ | 3,2 |
The temperature of the container is now increased to 350 K.
When a NEW equilibrium is established, it is found that 1,2 mol P(g) is present in the container.
6.2.1 Is the heat of the reaction (ΔH) POSITIVE or NEGATIVE? (1)
6.2.2 Use Le Chatelier's principle to explain the answer to QUESTION 6.2.1. (3)
6.2.3 Calculate the equilibrium constant at 350 K. (8)
6.2.4 How will the equilibrium constant calculated in QUESTION 6.2.3 be affected when the volume of the container is decreased at constant temperature?
Choose from INCREASES, DECREASES or REMAINS THE SAME.
Give a reason for the answer. (2)
6.3 More Q2(g) is now added to the reaction mixture at constant temperature.
How will EACH of the following be affected?
Choose from INCREASES, DECREASES or REMAINS THE SAME.
6.3.1 The yield of PQ(g) (1)
6.3.2 Number of moles of P(g) (1)
[18]
QUESTION 7 (Start on a new page.)
7.1 Sulphuric acid, H2SO4, ionises into two steps as follows:
- H2SO4(aq) + H2O(ℓ) → HSO 4 (aq) + H3O+(aq) Ka = 1 x 103
- HSO4 (aq) + H2O(ℓ) → SO2 4 (aq) + H3O+(aq) Ka = 1 x 10-2
7.1.1 Define an acid in terms of the Lowry-Brønsted theory. (2)
7.1.2 Write down the NAME or FORMULA of the substance that acts as an ampholyte in the above equations.
Give a reason for the answer. (2)
7.1.3 The conductivity of solutions of HSO 4 (aq) and H2SO4(aq) are compared. Which solution will have a LOWER conductivity?
Explain the answer. (3)
7.2 The pH of a hydrochloric acid solution, HCℓ(aq), is 1,02 at 25 °C.
7.2.1 Calculate the concentration of the HCℓ(aq). (3)
This HCℓ solution reacts with sodium carbonate, Na2CO3, according to the following balanced equation:
2HCℓ(aq) + Na2CO3(aq) → 2NaCℓ(aq) + CO2(g) + H2O(ℓ)
50 cm3 of the HCℓ solution is added to 25 cm3 of a 0,075 mol∙dm-3 Na2CO3 solution.
7.2.2 Calculate the concentration of the EXCESS HCℓ in the new solution. (8)
[18]
QUESTION 8 (Start on a new page.)
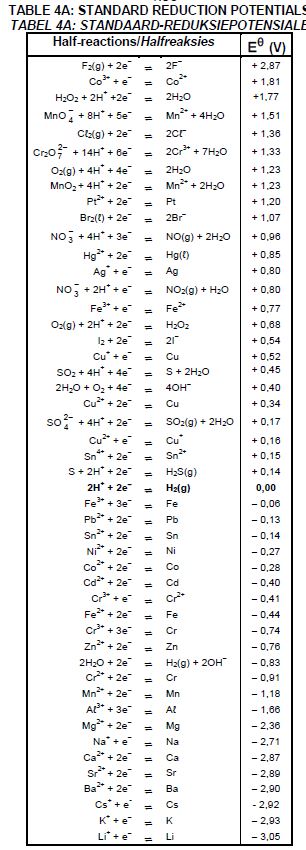
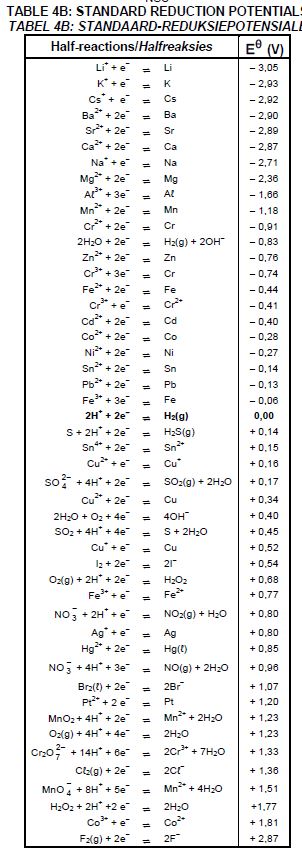
The table below shows two half-cells, A and B, used to assemble an electrochemical cell under STANDARD CONDITIONS.
| Half-cell A | Cu2+ (aq) | Cu(s) |
| Half-cell B | Ag+ (aq) | Ag(s) |
8.1 State the energy conversion that takes place in this cell. (1)
8.2 Calculate the mass of silver nitrate, AgNO3, used to prepare 150 cm3 of the electrolyte solution in half-cell B. (4)
8.3 Define the term reducing agent. (2)
8.4 Write down the:
8.4.1 NAME or FORMULA of the reducing agent (1)
8.4.2 Balanced equation for the reaction that takes place(3)
8.5 Calculate the initial emf of this cell.(4)
8.6 How will the emf of the cell be affected if the concentration of the copper ions in half-cell A increases?
Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
[16]
QUESTION 9 (Start on a new page.)
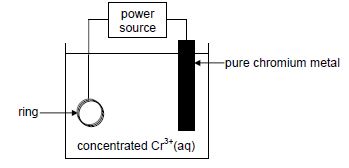
The diagram below shows a simplified electrolytic cell used to electroplate a ring.
9.1 Define the term electrolyte. (2)
9.2 Is the pure chromium metal the ANODE or the CATHODE of the cell? Give a reason for the answer. (2)
9.3 Write down the half-reaction that takes place at the ring. (2)
9.4 Calculate the total charge transferred when the mass of the pure chromium changes by 2 g. (5)
[11]
TOTAL: 150
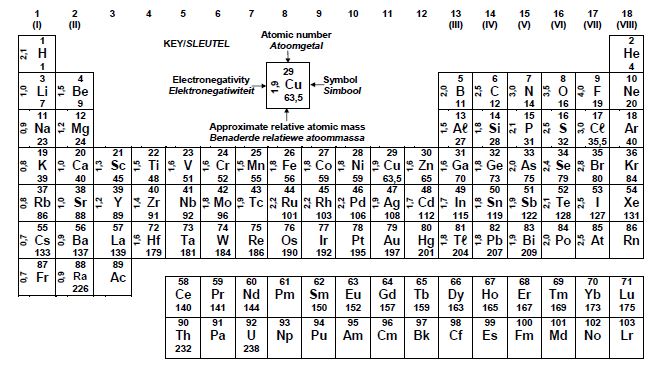
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
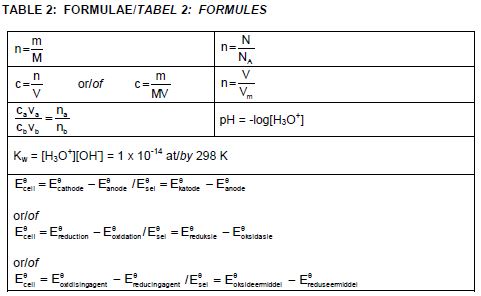
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | p° | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | T | 273 K |
| Charge on electron | e | -1,6 x 10-19 C |
| Avogadro's constant | NA | 6,02 x 1023 mol-1 |