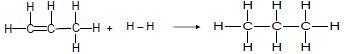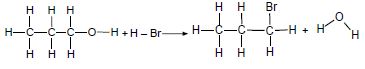TECHNICAL SCIENCES PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS NOVEMBER 2021
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupINSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of SIX questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, e.g. between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You are advised to use the attached DATA SHEETS.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, etc. where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.5) in the ANSWER BOOK, e.g. 1.6 D.
1.1 Consider the structural formula of a compound below and identify the type of homologous series to which the compound belongs:
- Carboxylic acid
- Aldehyde
- Alcohol
- Ketone
(2)
1.2 Study the table below and answer the following question.
| MOLECULAR NAME | BOILING POINT (°C) |
| Methane | - 164 |
| Ethane | - 89 |
| Propane | - 42 |
| Butane | - 0,5 |
Which ONE of the above molecules has the LOWEST vapour pressure?
- Methane
- Propane
- Ethane
- Butane
(2)
1.3 Which ONE of the following combinations is TRUE about the substance that is oxidised?
| ELECTRONS | OXIDATION NUMBER | |
| A | Gain | Decreases |
| B | Loss | Decreases |
| C | Gain | Increases |
| D | Loss | Increases |
1.4 An electrolyte conducts electricity because:
- It is a solid and ions are free to move.
- It is molten and ions are not moving.
- It is a solution and ions are free to move.
Which ONE of the combinations below is CORRECT?- (ii) only
- (i) and (ii)
- (iii) only
- (i), (ii) and (iii)
(2)
1.5 Which ONE of the following is CORRECT about the change in mass of electrodes in a galvanic cell? Assume that both electrodes are solid metals.
| ANODE | CATHODE | |
| A | Decreases | Increases |
| B | Decreases | Decreases |
| C | Increases | Increases |
| D | Increases | Decreases |
(2)
[10]
QUESTION 2 (Start on a new page.)
Organic molecules from different homologous series are represented in TABLE 1 below.
| A | CH3CH2CH2CH3 | B | 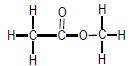 |
| C | CH3CH2 CH2CH2Cℓ | D | 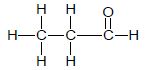 |
| E | Butan-1-ol | F | 2-chloropropane |
2.1 Define the term hydrocarbon. (2)
2.2 Draw the structural formula of the compounds represented by the following letters:
2.2.1 C (2)
2.2.2 E (2)
2.3 Write down the IUPAC name of compound B. (2)
2.4 Write down the homologous series to which the following compounds belong:
2.4.1 A (1)
2.4.2 B (1)
2.5 Compounds B and D are structural isomers.
2.5.1 Define the term structural isomer. (2)
2.5.2 What type of structural isomer are compounds B and D? (1)
[13]
QUESTION 3 (Start on a new page.)
Consider the compounds represented in TABLE 2 below and answer the questions that follow.
| A | 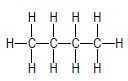 | B | 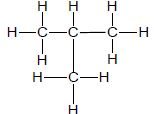 |
| C | 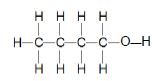 | D | 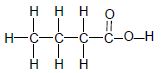 |
TABLE 2
3.1 Consider only compounds A and B.
3.1.1 What type of intermolecular forces exist between the molecules of these compounds? (1)
3.1.2 Which compound, A or B, has stronger intermolecular forces? (1)
3.1.3 Give a reason for the answer to QUESTION 3.1.2. (2)
3.2 The boiling point of compound A is compared to that of compound C.
3.2.1 Why is this a fair comparison? (2)
3.2.2 Which compound has a higher boiling point? (Write down only A or C.) (1)
3.2.3 Explain the answer to QUESTION 3.2.2. (3)
3.3 Arrange compounds A, B, C and D according to a decrease in vapour pressure. (2)
[12]
QUESTION 4 (Start on a new page.)
Consider the two reactions below and answer the questions that follow.
Reaction 1
Reaction 2
4.1 Write down the NAME of:
4.1.1 Reaction 1 (1)
4.1.2 Reaction 2 (1 )
4.2 Write down the following:
4.2.1 The NAME or FORMULA of a catalyst used in reaction 1 (1)
4.2.2 ONE reaction condition for reaction 2 (1)
4.3 The product in reaction 1 (propane) can react with bromine or oxygen.
4.3.1 Write down a balanced chemical equation for the reaction of propane and bromine using STRUCTURAL FORMULAE. (4)
4.3.2 Write down the NAMES or FORMULAE of TWO products formed when propane reacts in excess oxygen. (2)
4.4 A semiconductor is a solid substance that has electrical conductivity between a conductor and an insulator. The conductivity of a semiconductor can be improved by adding an impurity.
4.4.1 Name the process that is used to improve the conductivity of a semiconductor. (1)
4.4.2 Define an intrinsic semiconductor. (2)
4.4.3 Distinguish between an n-type semiconductor and a p-type semiconductor.(4)
[17]
QUESTION 5 (Start on a new page.)
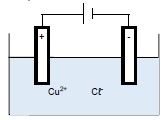
The diagram below represents an electrolytic cell used for the decomposition of copper(ll)chloride. Graphite electrodes are used in this cell.
5.1 For the electrolyte used in the cell above, write down the NAME of a/an:
5.1.1 Cation (1)
5.1.2 Anion (1)
5.2 Which electrode represents the following?
5.2.1 An anode (1)
5.2.2 A cathode (1)
5.3 Define the term oxidation. (2)
5.4 Write down a balanced half-reaction that occurs at the positive electrode. (2)
5.5 Define the term reducing agent. (2)
5.6 Draw a diagram showing a cell that is used to electroplate a spoon with silver using a battery. Indicate:
- The name of the electrolyte
- Anode and cathode in terms of a spoon and silver electrode
- A battery (3)
[13]
QUESTION 6 (Start on a new page.)
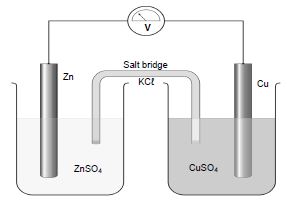
6.1 Learners performed an experiment to determine the electrode potential of a zinc-copper cell. They assembled the apparatus as shown in the diagram below. The experiment was performed under standard conditions.
6.1.1 Which type of cell is represented by the diagram above? (1)
6.1.2 Write down a balanced equation for the net ionic reaction of the above cell. (2)
6.1.3 Calculate the emf of the cell. (4)
6.2 Consider the two reactions below and answer the questions that follow.
Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s) Reaction A
Ni(s) + ZnSO4(aq) → NiSO4(aq) + Zn(s) Reaction B
6.2.1 Which ONE of the above reactions is spontaneous?
Write down REACTION A or REACTION B only. (1)
6.2.2 Motivate the answer to QUESTION 6.2.1 by referring to the reducing ability of the reactants. (2)
[10]
TOTAL: 75