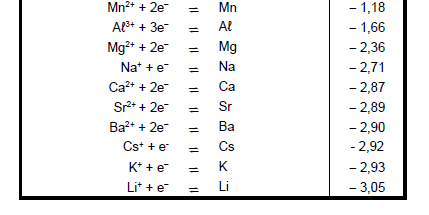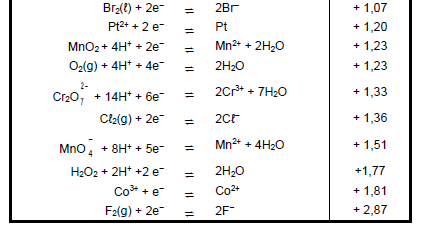PHYSICAL SCIENCES PAPER 2 GRADE 12 - NSC PAST PAPERS AND MEMOS SEPTEMBER 2016
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES PAPER 2
GRADE 12
NATIONAL SENIOR CERTIFICATE
SEPTEMBER 2016
INSTRUCTIONS AND INFORMATION
- Write your full NAME and SURNAME in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number your answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two sub questions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS and PERIODIC TABLE.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Four possible options are provided as answers to the following questions. Each question has only ONE correct answer. Write only the correct letter (A to D) next to the question number (1.1 to 1.10) in the ANSWER BOOK, for e.g. 1.11 E.
1.1 The temperature at which the solid and liquid phases of a substance are at equilibrium is known as the …
- boiling point.
- melting point.
- change in enthalpy.
- standard temperature. (2)
1.2 Which ONE of the following compounds represents the first member of the ketones?
- HCHO
- CH3OH
- H3COCH3
- CH3CH2COOH (2)
1.3 The following reaction is a Lowry-Bronsted acid base reaction. NH3(g) + HCℓ(g) ⇋ NH4Cℓ(g)
- The reason the reaction is classified as an acid base reaction is that …
- NH3 accepts a proton.
- HCℓ accepts a proton.
- NH3 donates a proton.
- HCℓ donates an electron. (2)
1.4 In which ONE of the following solutions will the concentration of the hydroxide ions [OH-] be less than 10-7 mol∙dm-3 at 25 oC?
- KCℓ(s) + H2O(ℓ) →K+(aq) + Cℓ-(aq)
- NaCℓ(s) + H2O(ℓ) →Na+(aq) + Cℓ-(aq)
- NH4Cℓ(s) + H2O(ℓ)→ NH4 +(aq) + Cℓ-(aq)
- Na2CO3(s) + H2O(ℓ) → 2Na+(aq) + CO3 2-(aq) (2)
1.5 Which ONE of the following compounds is the final product in the contact process?
- NH3
- HNO3
- H2SO4
- (NH4)2SO4 (2)
1.6 The reaction represented by the equation below reaches equilibrium in a sealed container:
CaCO3(s) → CaO(s) + CO2(g) ΔH >0
The pressure on the system is increased by decreasing the volume at constant temperature. How does the concentration of CO2, [CO2], and the equilibrium constant Kc change when equilibrium is re-established?
| [CO2] | Kc | |
| A | Remains constant | Remains constant |
| B | Higher | Higher |
| C | Lower | Remain constant |
| D | Lower | Lower |
(2)
1.7 Three catalysts are used separately to increase the rate of a hypothetical reaction. In the diagram below, E1, E2 and E3 represent the effect of each catalyst on the activation energy (E0) for the reaction.
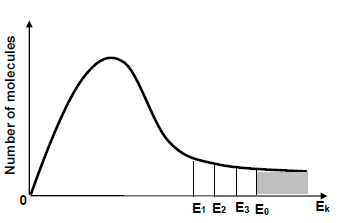
Which ONE of the following is the activation energy for the reaction with the HIGHEST rate?
- E1
- E2
- E3
- E0 (2)
1.8 Which ONE of the following is a PRODUCT of the HYDROGENATION of ethene? (2)
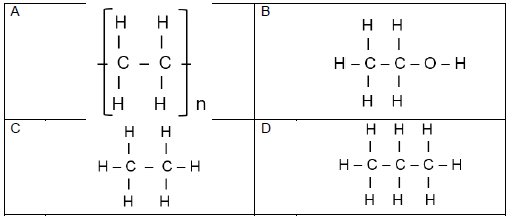
1.9 Which ONE of the following correctly defines electrolysis?
- Decomposition of an ionic compound by using heat.
- The use of electrical energy to produce a chemical change.
- A solution or liquid that conducts electricity through the movement of ions.
- The chemical process in which chemical energy is converted to electrical energy. (2)
1.10 Consider the electrochemical cell based on the following reaction:
2Cr3+(aq) + Cr(s) → 3Cr2+
The table below describes the concentration of Cr2+ ions at the electrodes, as well as the movement of anions in the cell.
Which ONE of the following combinations is CORRECT when the cell delivers electric current?
| Concentration of [Cr2+] at cathode | Concentration of [Cr2+] at anode | Movement of anions | |
| A | Decreases | Increases | Towards anode |
| B | Decreases | Decreases | Towards cathode |
| C | Increases | Increases | Towards both anode and cathode |
| D | Increases | Increases | Towards anode |
(2)
[20]
QUESTION 2 (Start on a NEW PAGE.)
The letters below A to F in the table below represent six organic compounds.
A 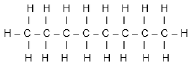 | B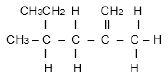 |
| C C3H7Cℓ | D Propanoic acid |
| E Polyethene | F CnH2nO2 |
Use the information in the table to answer the questions that follow:
2.1 Write down the letter of the compound that …
2.1.1 has a carboxyl group. (1)
2.1.2 is used to make plastic. (1)
2.2 Write down the …
2.2.1 IUPAC name of compound B. (2)
2.2.2 STRUCTURAL FORMULA of the monomer of compound E. (2)
2.3 Compound A is an alkane. Write down the …
2.3.1 GENERAL FORMULA for alkanes. (1)
2.3.2 MOLECULAR FORMULAE of each of the two products obtained during the complete combustion of compound A. (2)
2.4 Compound C is a primary haloalkane:
2.4.1 Write down the STRUCTURAL FORMULA and IUPAC name of a STRUCTURAL ISOMER of compound C. (4)
2.4.2 Classify the isomer in QUESTION 2.4.1 as CHAIN, POSITIONAL or FUNCTIONAL. (1)
2.5 Chemical analysis of compound F shows that it has the following percentage composition: x% carbon (C), y% hydrogen (H) and 12,5% oxygen (O).
Use a calculation to determine the value of x. (4)
[18]
QUESTION 3 (Start on a NEW PAGE.)
3.1 The flow diagram below shows two organic reactions in which 2-chlorobutane reacts with potassium hydroxide (KOH) under different reaction conditions.
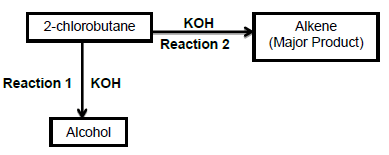
Use the information in the flow diagram to answer the questions that follow:
3.1.1 Write down the …
- type of reaction of which Reaction 1 is an example. (1)
- IUPAC name of the alcohol. (2)
3.1.2 Which ONE of the reactions, 1 or 2, uses concentrated potassium hydroxide? (1)
3.1.3 Write down the STRUCTURAL FORMULA of the alkene. (2)
3.2 A small sample of propyl methanoate is prepared in a school laboratory using an alcohol and a carboxylic acid in the presence of a catalyst. The reaction mixture is heated in a water bath. Write down the …
3.2.1 reason why the reaction mixture is heated in a water bath instead of heating directly. (1)
3.2.2 STRUCTURAL FORMULA of the alcohol. (2)
3.2.3 IUPAC name of the carboxylic acid. (1)
[10]
QUESTION 4 (Start on a NEW PAGE.)
There are three chain isomers having the molecular formula, C5H12. In a practical investigation, vapour pressure data for the three chain isomers A, B and C is collected and plotted on a graph as shown below.
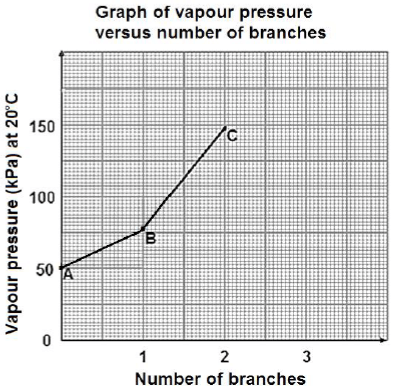
4.1 Define the term chain isomer. (2)
4.2 Use the graph to estimate the vapour pressure of the straight chain isomer of C5H12 at 20ºC. (1)
4.3 Write down the STRUCTURAL FORMULA of compound C. (2)
4.4 Explain the difference in the vapour pressure of compound A and B. In your explanation refer to the STRUCTURE of the molecules, the TYPE and STRENGTH of the INTERMOLECULAR FORCE(S). (3)
4.5 The learners also collected boiling point data for compounds D, E and F as shown in the table below.
| Compound | Condensed Structural Formula | Boiling point (°C) |
| D | CH3OH | 78 |
| E | CH3CH2CH2OH | 97 |
| F | CH3Cℓ2 | 39,6 |
4.5.1 Write down the NAME of the type of intermolecular force that is responsible for the difference in the boiling points of compound D and E. (2)
4.5.2 Explain the difference in the boiling points of compounds D and F by referring to the TYPE and STRENGTH of intermolecular forces. (3)
Compound F is prepared at standard conditions (STP) by the reaction between methane and chlorine as shown by the equation:
CH4(g) + Cℓ2(g) → CH2Cℓ2(g) + H2(g)
4.5.3 Write down the NAME of the type of reaction that leads to the formation of compound F. (1)
4.5.4 In the reaction 21,88 dm3 of CH4 produces 0,043 kg of CH2Cℓ2. Calculate the percentage yield in this reaction. (5)
[19]
QUESTION 5 (Start on a NEW PAGE.)
Learners investigate some of the factors that influence the rate of a chemical reaction. In the experiment they add equal amounts of each of three different metals separately to equal volumes of EXCESS dilute hydrochloric acid solution.
In each experiment the acid completely covers the metal.
The data obtained is recorded as in the table below:
| Experiment | Amount of metal powder | Change in temperature of solution (°C) (Tfinal –Tinitial) | Time taken to run to completion |
| 1 | 0,1 mol Zn | +23 | 25,2 |
| 2 | 0,1 mol Mg | +37 | 8,3 |
| 3 | 0,1 mol Cu | 0 | No reaction |
5.1 Is the reaction in Experiment 1 ENDOTHERMIC or EXOTHERMIC? Give a reason for your answer. (Use the information in the table). (2)
5.2 Which factor influencing reaction rate is investigated? (1)
5.3 How will the total volume of hydrogen gas produced in Experiment 2 compare with the total volume of hydrogen gas produced in Experiment 1 at the end of the reactions?
Write down HIGHER THAN, EQUAL TO or SMALLER THAN.
Give a reason for your answer. (2)
5.4 The graphs obtained for Experiment 1 and Experiment 2, labelled as 1 and 2 respectively, are sketched on the same set of axes as shown below:
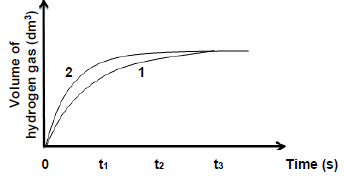
5.4.1 In which experiment does the reaction occur at a higher reaction rate at time t1? (1)
5.4.2 Explain the answer to QUESTION 5.4.1 by referring to the relative strength of reducing agents involved. (2)
5.5 In another experiment, Experiment 4, the same reaction conditions are repeated as in Experiment 2, but the reaction mixture is heated. The rate of reaction is HIGHER for Experiment 4 than 2.
Explain why the reaction rate is HIGHER for Experiment 4 than 2 by referring to the collision theory. (3)
[11]
QUESTION 6 (Start on a NEW PAGE.)
The reaction represented by the equation below reaches equilibrium in a closed container.
N2(g) + 3H2(g) ⇋ 2NH3(g) ΔH = -92 kJ
6.1 Is the above equilibrium HOMOGENEOUS or HETEROGENEOUS? Give a reason for the answer. (2)
6.2 Changes were made to the TEMPERATURE, PRESSURE and CONCENTRATION of the above equilibrium mixture. The graphs below represent the results obtained.
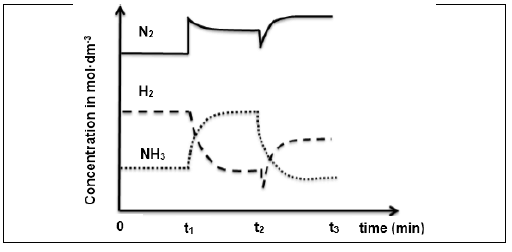
6.2.1 What changes were made to the reaction conditions at each of the following times?
- t1 (1)
- t2 (1)
6.2.2 How does the rate of the forward reaction compare to the rate of the reverse reaction between 0 and t1? Write down HIGHER THAN, LOWER THAN or EQUAL TO. (1)
6.3 Equal number of moles of hydrogen gas and nitrogen gas are injected into a sealed 1 dm3 container. When the reaction reaches equilibrium at temperature T1 it is found that 10% of the original amount of hydrogen is left in the container. The value of Kc at temperature T1 is 1,426 × 103.
6.3.1 Calculate the initial mass of N2 in the container. (10)
6.3.2 Use your knowledge of Le Chatelier’s principle to explain how an increase in temperature will affect the value of Kc. (3)
[18]
QUESTION 7 (Start on a NEW PAGE.)
Learners perform a titration to standardise a dilute sodium hydroxide (NaOH) solution. They use standard oxalic acid (H2C2O4.2H2O) solution of concentration 0,02 mol∙dm-3. The titration is repeated three times after which the average volume readings are calculated as shown in the table below:
| Titrations | Volume of oxalic acid solution (cm3) | Volume NaOH solution (cm3) |
| 1 | 25 | 20,24 |
| 2 | 25 | 19,80 |
| 3 | 25 | 19,87 |
| Average | 25 | 19,97 |
7.1 What does the term standard solution mean? (2)
7.2 Give a reason why the titration is repeated three times. (1)
7.3 The balanced equation for the reaction taking place is:
H2C2O4.2H2O(aq) + 2NaOH(aq) → Na2C2O4(aq) + 4H2O(ℓ)
Calculate the number of moles of oxalic acid reacting. (3)
7.4 The dilute solution of sodium hydroxide used in the titration was obtained by adding 90 cm3 of water to 10 cm3 of a sodium hydroxide solution.
Calculate the pH of the sodium hydroxide solution BEFORE dilution. (8)
[14]
QUESTION 8 (Start on a NEW PAGE.)
8.1 Consider the electrochemical cell below. Electrode Y in half cell B is an inert metal
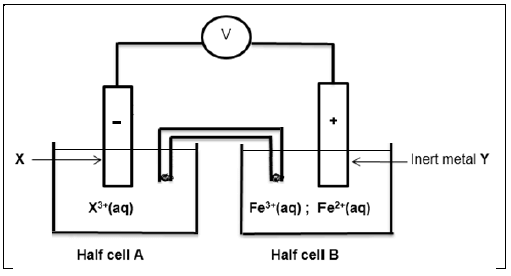
For the electrochemical cell, write down …
8.1.1 ONE function of the saltbridge. (1)
8.1.2 the voltmeter reading when the cell reaction reaches equilibrium. (1)
8.1.3 name or formula of the inert metal Y. (1)
8.1.4 name or formula of the oxidising agent. (1)
8.1.5 half reaction taking place on the surface of electrode Y. (2)
8.2 The initial EMF of the above electrochemical cell under standard conditions is 0,83 V.
Identify metal X by calculation. (5)
8.3 Write down the cell notation for this cell. (3)
[14]
QUESTION 9 (Start on a NEW PAGE.)
9.1 Two different cells, A and B are shown in the diagrams below. Cell A contains a concentrated solution of sodium chloride (NaCℓ) and cell B contains a concentrated solution of copper(II) chloride (CuCℓ2). P, Q, R and S are identical carbon electrodes.
Chlorine gas is formed at electrode P and S.
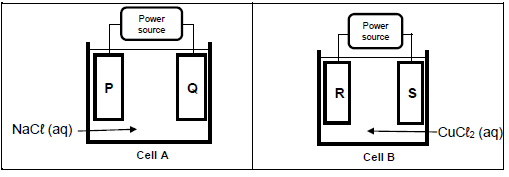
9.1.1 Is cell A ELECTROLYTIC or GALVANIC? Give a reason for your answer. (2)
9.1.2 Write down the equation for the half reaction taking place at electrode Q. (2)
9.1.3 Write down the NAME or SYMBOL of the product formed at electrode R. (1)
9.1.4 What happens to the concentration of the electrolyte in cell B when the cell is in operation?
Write down INCREASES, DECREASES or REMAINS THE SAME. Give a reason for the answer. (3)
9.2 Aluminium is obtained from alumina (Aℓ2O3) using an electrolytic process. In the process, cryolite (Na3AℓF6), is added to alumina in order to reduce the melting point of alumina.
9.2.1 Give a reason why it is necessary to melt alumina before electrolysis. (1)
9.2.2 The electrolyte in the cell contains Na+ ions. Refer to the attached Table of Standard reduction Potentials to explain why the Na+ ions will not affect the purity of the aluminium obtained during this process. (3)
[12]
QUESTION 10 (Start on a new page.)
The flow diagram below shows the steps involved in the industrial preparation of ammonia and nitric acid.
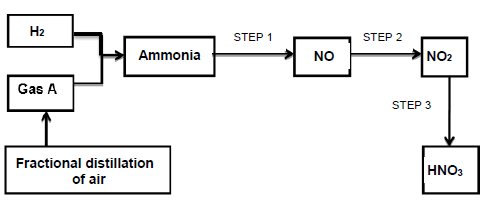
10.1 Write down the name of …
10.1.1 gas A. (1)
10.1.2 the catalyst used in the preparation of ammonia. (1)
10.2 The reaction in STEP 2, reaches equilibrium according to the equation below:
2NO(g) + O2(g) ⇋ 2NO2(g) ΔH = -149,1 kJ
Calculate the amount of the net energy released (in kJ) by the reaction when
1,12 g of the initial amount of NO(g) is used up. (4)
10.3 Nitric acid reacts with ammonia to produce a fertiliser.
10.3.1 Classify the reaction taking place as PROTOLYTIC or REDOX. (1)
10.3.2 Write down a balanced equation for the reaction. (3)
10.4 A 50 kg bag of N:P:K fertiliser is labelled 7:1:3(60). Calculate the mass of potassium in the bag of fertiliser. (3)
10.5 Name ONE negative effect of the overuse of nitrogen based fertilisers on the environment. (1)
[14]
TOTAL: 150
NATIONAL SENIOR CERTIFICATE
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | e -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m n = N n = V | c = n V or c = m MV caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | ||
| Eθ cell = Eθ cathode – Eθ anode / E Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |
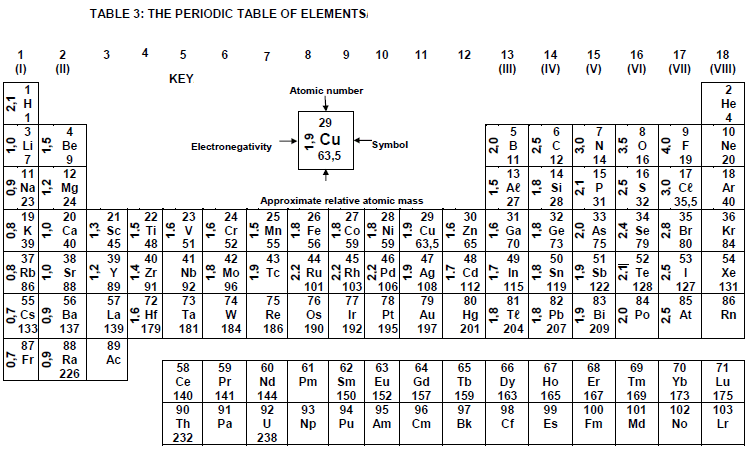
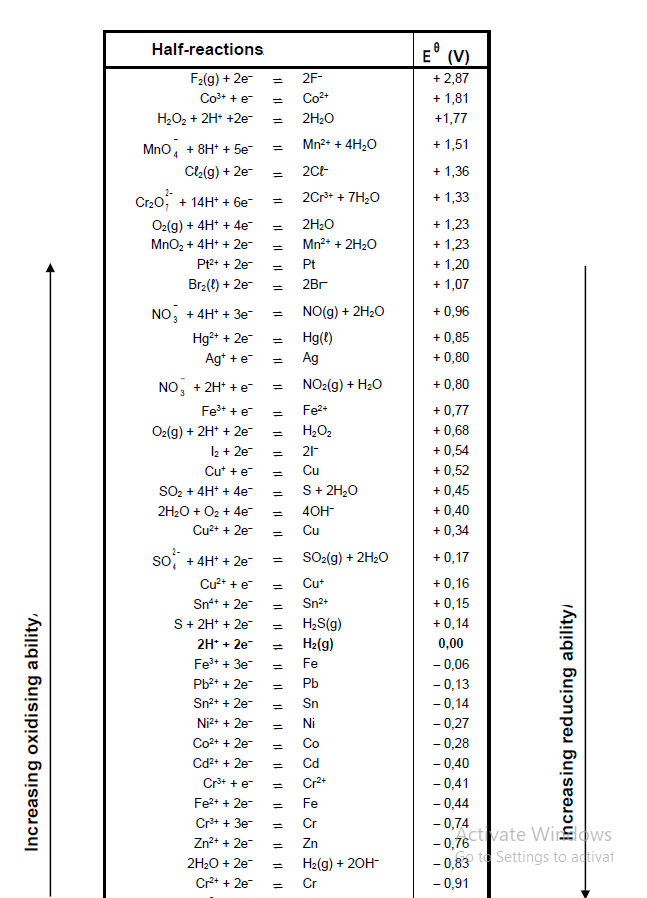
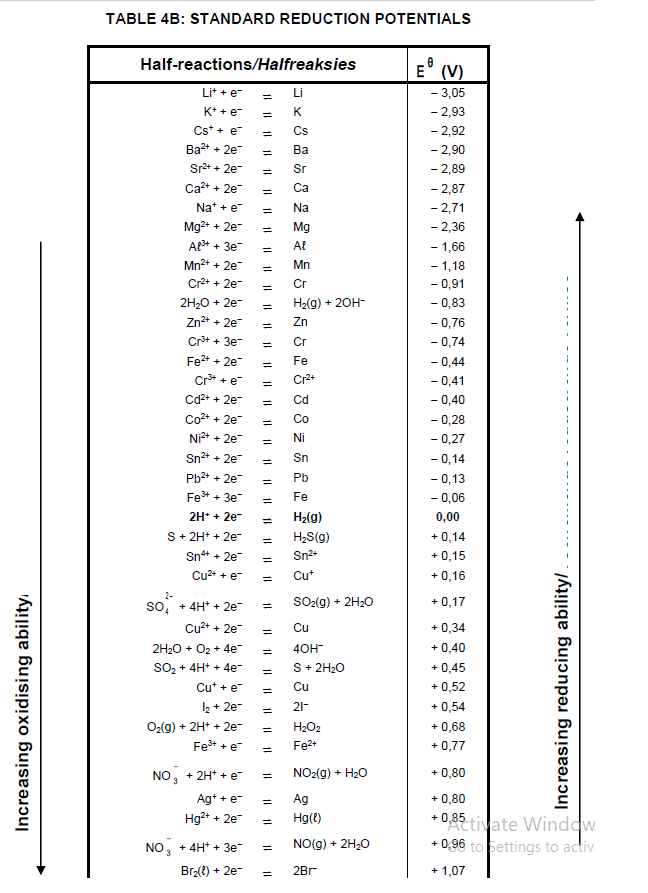
TABLE 4A: STANDARD REDUCTION POTENTIALS