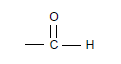PHYSICAL SCIENCES: (CHEMISTRY) P2 with Memorandum - 2024 Grade 12 June Common Exams
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupMARKS: 150
TIME: 3 hours
INSTRUCTIONS AND INFORMATION
1. Write your name and surname in the appropriate space on the ANSWER BOOK.
2. This question paper consists of SEVEN questions. Answer ALL the questions in the ANSWER BOOK.
3. Start EACH question on a NEW page in the ANSWER BOOK.
4. Number the answers correctly according to the numbering system used in this question paper.
5. Leave ONE line between two sub-questions, for example between QUESTION 2.1 and QUESTION 2.2.
6. You may use a non-programmable calculator.
7. You may use appropriate mathematical instruments.
8. Show ALL formulae and substitutions in ALL calculations.
9. Round off your FINAL numerical answers to a minimum of TWO decimal places.
10. Give brief motivations, discussions, et cetera. where required. 11. You are advised to use the attached DATA SHEETS.
12. Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.10) in the ANSWER BOOK, for example 1.11 E.
1.1 The homologous series that contain a carbon-carbon triple bond is …
A alkanes.
B alkenes.
C alkynes.
D haloalkanes. (2)
1.2 Consider the following compounds:
Compounds
A | Pentan-1-ol |
B | Butan-1-ol |
C | Pentanoic acid |
Which ONE of the following correctly rank the above pure substances in the order of increasing strength of intermolecular forces?
A Pentan-1-ol, butan-1-ol, pentanoic acid
B Pentanoic acid, butan-1-ol, pentan-1-ol
C Butan-1-ol, pentanoic acid, pentan-1-ol
D Butan-1-ol, pentan-1-ol, pentanoic acid (2)
1.3 Consider the structural formula:
Which ONE of the following compounds contains the above functional group?
A Propanal
B Propanone
C Propan-1-ol
D Propanoic acid (2)
1.4 2-methylpropan-1-ol can form two isomers. Which ONE of the following combinations CORRECTLY identifies the ISOMER and the TYPE OF ISOMER?
NAME OF ISOMER | TYPE OF ISOMER | |
A | Butan-1-ol | Positional |
B | 2-methylpropan-2-ol | Chain |
C | Butan-1-ol | Functional |
D | 2-methylpropan-2-ol | Positional |
1.5 The conversion of CH3CHCH2 to CH3CH2CH3 is known as …
A hydration.
B hydrogenation.
C halogenation.
D hydrohalogenation. (2)
1.6 Hydrochloric acid reacts with EXCESS zinc according to the balanced equation:
2 HCℓ (aq) + Zn (s) → ZnCℓ2 (aq) + H2 (g)
Which ONE of the following factors will influence the yield of H2 (g) but not on the rate of production of H2 (g)?
A Temperature
B Volume of HCℓ
C State of division of Zn
D Concentration of HCℓ (2)
1.7 Carbonic acid, H2CO3, ionises in water in two steps. The first step of the ionisation is given by the equation:
H2CO3 (aq) + H2O (ℓ) ⇌ HCO3 - (aq) + H2O+(aq)
Which ONE of the following substances in the above reaction can act as an ampholyte?
A H2CO3 and H2O
B HCO3 - and H3O+
C H2O and HCO3 -
D H2CO3 and HCO3 - (2)
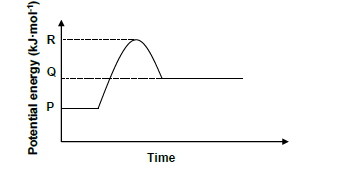
1.8 The potential energy diagram for the following reversible hypothetical reaction is given:
2 AB (g) ⇌ A2 (g) + Br2 (g)
Consider the following statements regarding the energy diagram.
I Δ H for the forward reaction is positive
II Catalyst would lower P–Q
III Reversible reaction is exothermic
Which of the statement(s) above is/are TRUE?
A I only
B I and II only
C I and III only
D II and III only (2)
1.9 Consider the following reversible reaction at equilibrium in a sealed container:
C (s) + CO2 (g) ⇌ 2 CO (g)
The volume inside the container is decreased while the temperature remains constant.
Which ONE of the following combinations are CORRECT regarding the amount of CO and the rate at which the new equilibrium is reached?
AMOUNT OF CO | REACTION RATE | |
A | Higher | Lower |
B | Lower | Higher |
C | Higher | Higher |
D | Lower | Lower |
(2)
1.10 Consider two solutions of Ba(OH)2 (aq) and KOH (aq) each with a concentration of 0,1 mol·dm-3.
Consider the following statements regarding the two solutions.
I Both KOH and Ba(OH)2 can be regarded as Arrhenius bases
II Ba(OH)2 will produce a higher concentration of OH–than KOH when it dissociates
III Double the amount of HCℓ is needed to neutralise KOH than Ba(OH)2
Which of the statement(s) above is/are TRUE?
A I only
B I and II only
C II and III only
D I and III only (2) [20]
QUESTION 2 (Start on a new page.)
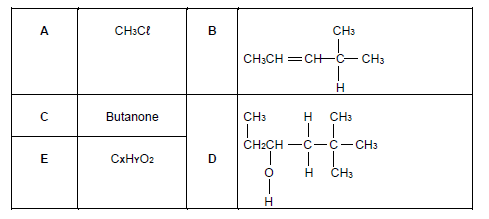
The table below shows organic molecules A to E from different homologous series.
2.1 Define homologous series. (2)
2.2 Write down the LETTER that represents the following compounds:
2.2.1 Hydrocarbon (1)
2.2.2 Haloalkane (1)
2.2.3 Alkene (1)
2.2.4 The compound that contains the carbonyl group that is bonded to two saturated carbon atoms (1)
2.3 Is compound D a PRIMARY, SECONDARY OR TERTIARY ALCOHOL? Give a reason for the answer. (2)
2.4 Write down the:
2.4.1 General formula for the homologous series to which compound B belong. (1)
2.4.2 IUPAC name of compound B (2)
2.4.3 IUPAC name of compound D (3)
2.5 Compound C has a functional isomer.
2.5.1 Define the term functional isomer. (2)
2.5.2 Draw the STRUCTURAL FORMULA of the functional isomer of compound C. (2)
2.6 Compound E (CXHYO2) reacts with alcohol P in the presence of concentrated sulphuric acid (H2SO4) to produce organic compound Q as shown by the incomplete equation below:
CXHYO2 + alcohol P → organic compound Q + H2O
The percentage composition of compound Q is:
Organic compound Q | ||
Carbon | Hydrogen | Oxygen |
58,82% | 9,81% | 31,37% |
The molecular mass of the compound Q is EQUAL to the formula mass.
2.6.1 Write down the name of the type of reaction that occurred. (1)
2.6.2 Determine, by calculation, the molecular formula of organic compound Q. (5)
Compound E (CXHYO2) has a molecular mass of 74 g·mol-1.
2.6.3 Determine the compound E (CXHYO2) and write down its IUPAC name. (4)
2.6.4 Determine the organic compound Q that was produced, write down its IUPAC name and STRUCTURAL FORMULA. (6) [34]
QUESTION 3 (Start on a new page.)
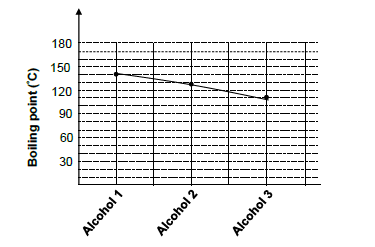
An investigation is carried out to determine the effect of branching on the boiling points of an organic compounds. Three PRIMARY ALCOHOLS that each contains 5 carbon atoms are used during this investigation.
Equal volumes of each alcohol are heated separately in a water bath.
3.1 Define boiling point. (2)
3.2 What property of alcohols requires them to be heated in a water bath? (1)
3.3 Write down the boiling point of alcohol 1. (1)
3.4 Is this a fair investigation? Write only YES or NO.Give a reason for the answer. (2)
3.5 Give the IUPAC name of alcohol 2. (2)
3.6 Which alcohol has the shortest chain length? Write down only ALCOHOL 1, ALCOHOL 2 or ALCOHOL 3. (1)
3.7 Fully explain the answer to QUESTION 3.6. (3)
3.8 A second investigation is carried out to determine the effect of intermolecular forces on the vapour pressure.
The table below summarises the results from two organic compounds.
COMPOUND | VAPOUR PRESSURE AT 20 °C (kPa) | |
A | Butanone | 9,47 |
B | Butan-1-ol | 0,58 |
3.8.1 Define vapour pressure. (2)
3.8.2 Explain the difference in vapour pressures by referring to the intermolecular forces involved. (4)
3.8.3 Will the vapour pressure of the above compounds INCREASE, DECREASE or REMAIN THE SAME at a higher temperature? (1) [19]
QUESTION 4 (Start on a new page.)
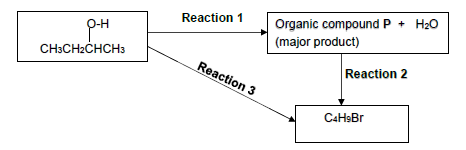
4.1 Consider the flow diagram showing organic reactions given below. Reaction 1
Consider REACTION 1.
Write down the:
4.1.1 Name of the type of elimination reaction (1)
4.1.2 Name or formula of the inorganic reagent needed (1)
4.1.3 Balanced equation using STRUCTURAL FORMULAE for the organic compounds (4)
Consider REACTION 2.
Write down the:
4.1.4 Name the type of reaction taking place (1)
4.1.5 STRUCTURAL FORMULA and IUPAC name for the major product formed (4)
Consider REACTION 3.
Write down the:
4.1.6 TWO reaction conditions needed (2)
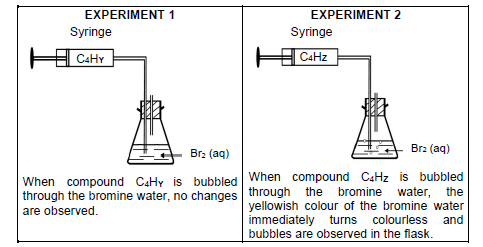
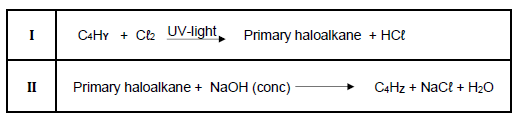
4.2 Octane can be cracked according to the incomplete equation:
C8H18 → C4 HY + C4 HZ
The two STRAIGHT CHAIN organic compounds, C4 HZ and C4 HY, are now passed through bromine water (Br2 (aq)) at room temperature in a darken room. The following observations are made:
4.2.1 Define cracking. (2)
4.2.2 Give a reason why experiments 1 and 2 is carried out in a darken room. (1)
4.2.3 Which compound, C4 HY or C4 HZ, is UNSATURATED? Give a reason for the answer.
Compound C4 HY undergoes the following reactions:
Write down the:
4.2.4 STRUCTURAL FORMULA for compound C4 HZ (2)
4.2.5 Combustion reaction of compound C4 HY using MOLECULAR FORMULAE (3) [23]
QUESTION 5 (Start on a new page.)
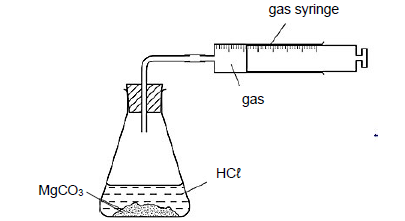
A group of learners use the reaction between magnesium carbonate (MgCO3) and EXCESS hydrochloric acid (HCℓ) to investigate some of the factors that affect the reaction rate. The balanced equation for the reaction is:
MgCO3 (aq) + 2 HCℓ (aq) MgCℓ2 (aq) + H2 O (ℓ) + CO2 (g)
The learners used the apparatus illustrated below.
The table below summarises the reaction conditions:
EXPERIMENT | REACTION CONDITIONS | ||
CONCENTRATION OF HCℓ (mol·dm-3) | STATE OF DIVISION OF MgCO3 | INITIAL TEMPERATURE (°C) | |
1 | 0,9 | Powder | 25 |
2 | 0,9 | Powder | 30 |
3 | 0,9 | Lumps | 30 |
5.1 Define reaction rate. (2)
5.2 Write down the independent variable for the comparison between experiment 1 and 2. (1)
5.3 Experiment 2 and 3 is now compared.
5.3.1 Which experiment, 2 or 3, will have the highest reaction rate? (1)
5.3.2 Explain the answer to QUESTION 5.3.1 by referring to the collision theory. (3)
5.4 The learners measured the rate at which CO3 was produced in experiment 2 and found it to be 0,25 g·min-1. It took 10,44 minutes to measure the time taken for the reaction to reach completion.
Calculate the:
5.4.1 Mass of MgCO3 that was used (6)
5.4.2 Molar volume of CO2 if 1,47 dm3 of CO2 was released (3)
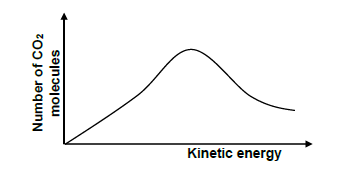
5.5 The graph below represents Maxwell-Boltzmann distribution curve for CO2(g) produced experiment 1.
Redraw the graph in your ANSWER BOOK. Clearly label the curve as A.
On the same set of axes, sketch the curve that will be obtained for CO2(g) if the mass of MgCO3 used is increased. Label this curve as B. (2) [18]
QUESTION 6 (Start on a new page.)
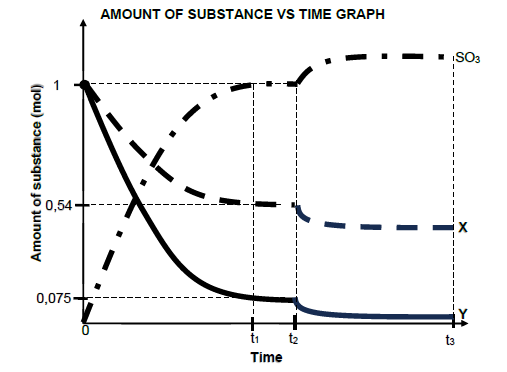
6.1 Initially 1 mol of sulphur dioxide SO2 (g) and oxygen O2 (g) are allowed to react in a sealed container according to the balanced equation:
2 SO2(g) + O2(g) ⇌ 2 SO3 (g)
The graph below shows the change in amounts of reactants and products over time.
Graph is NOT drawn to scale.
6.1.1 State Le Chatelier’s principle in words. (2)
6.1.2 How will the rate of the forward reaction compare to the rate of the reverse reaction between t1 and t2?
Choose from HIGHER THAN, LOWER THAN or EQUAL TO.
Give a reason for the answer. (2)
6.1.3 Which curve, X or Y, represent SO2?
Give a reason for the answer. (2)
The temperature of the reaction mixture was decreased at t2.
6.1.4 Is the heat of the reaction (ΔH) POSITIVE or NEGATIVE for the forward reaction? (1)
6.1.5 Explain the answer to QUESTION 6.1.4 by referring to Le Chatelier’s principle. (3)
6.2 2,5 mol of NOCℓ was initially placed in a 1,5 dm3 sealed container at 400 °C. After the equilibrium was established, it was found that 28% of the NOCℓ dissociated according to the balanced equation:
2 NOCℓ (g) ⇌ 2 NO (g) + Cℓ2 (g)
6.2.1 Calculate the equilibrium constant, Kc-value at 400 °C. (7)
6.2.2 More NOCℓ is added to the equilibrium mixture. How will this change affect the equilibrium constant, Kc?
Write down only INCREASES, DECREASES or REMAINS THE SAME.
Give a reason for your answer. (2) [19]
QUESTION 7 (Start on a new page.)
7.1 The balanced equation below represents the first step of the ionisation reaction of sulphuric acid (H2 SO4):
H2 SO (aq) + H2 O(ℓ) ⇌ HSO4 -(aq) + H3 O+(aq)
7.1.1 Define an acid according to the Arrhenius theory. (2)
Write down the:
7.1.2 FORMULAE of the TWO BASES in the above reaction (2)
7.1.3 Balanced chemical equation for the reaction between sulphuric acid (H2 SO4) and potassium hydroxide (KOH) (3)
7.2 A standard solution of sodium hydroxide (NaOH) is prepared by dissolving 3,812 g to make a 100 cm3 NaOH solution.
7.2.1 Calculate the concentration of sodium hydroxide (NaOH) solution. (3)
Household vinegar contains x % ethanoic acid (CH3COOH) by mass. 25 cm3 of vinegar reacts with 21,8 cm3 sodium hydroxide (NaOH) solution prepared in QUESTION 7.2.1.
The balanced equation is:
CH3COOH (aq) + NaOH (aq) → CH3COONa (aq) + H2O(ℓ)
7.2.2 Calculate the percentage mass of the ethanoic acid (value of x) found in the vinegar if 1 cm3 of household vinegar has a mass of 1 g. (7) [17]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS/TABEL 1: FISIESE KONSTANTES
NAME/NAAM | SYMBOL/SIMBOOL | VALUE/WAARDE |
Standard pressure Standaarddruk | θ p | 1,013 x 105 Pa |
Molar gas volume at STP Molêre gasvolume teen STD | Vm | 22,4 dm3∙mol-1 |
Standard temperature Standaardtemperatuur | Tθ | 273 K |
Charge on electron Lading op elektron | e | -1,6 x 10-19 C |
Avogadro’s constant Avogadro se konstante | NA | 6,02 x 1023 mol-1 |
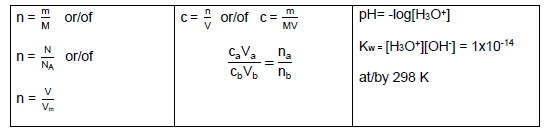
TABLE 2: FORMULAE/TABEL 2: FORMULES
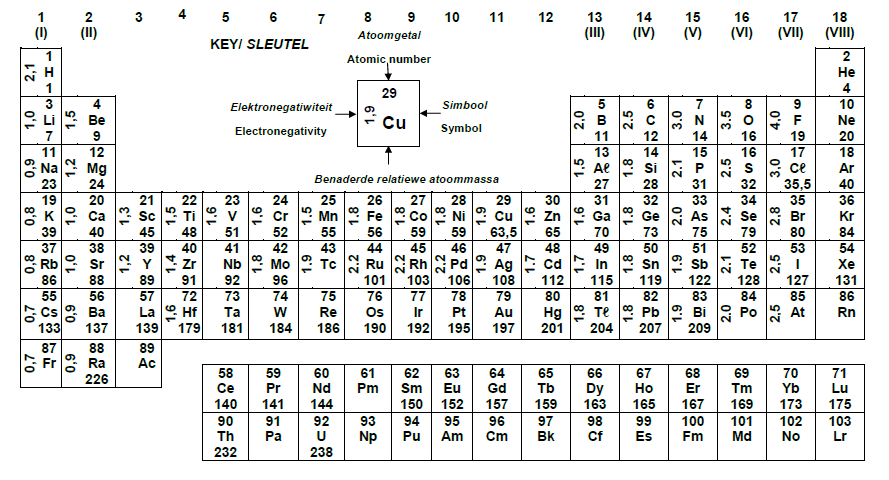
TABLE 3: THE PERIODIC TABLE OF ELEMENTS/TABEL 3: DIE PERIODIEKE TABEL VAN ELEMENTE