TECHNICAL SCIENCES: CHEMISTRY P2 with Memorandum - 2024 Grade 12 June Common Exams
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupMARKS: 75
TIME: 1½ hours
INSTRUCTIONS AND INFORMATION
1. Write your FULL NAME and SURNAME in the appropriate spaces in the ANSWER BOOK.
2. This question paper consists of SIX questions. Answer ALL the questions.
3. Start each question on a NEW page in the ANSWER BOOK.
4. Number the answers correctly according to the numbering system used in this question paper.
5. Leave ONE line between two sub-questions, for example between QUESTION 2.1 and QUESTION 2.2.
6. You may use a non-programmable calculator.
7. You are advised to use the attached DATA SHEETS.
8. Round off your FINAL numerical answers to a minimum of TWO decimal places.
9. You may use appropriate mathematical instruments.
10. Show ALL formulae and substitutions in ALL calculations.
11. Give brief motivations, discussions et cetera where required.
12. Write neatly and legibly.
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A–D) next to the question numbers (1.1 to 1.5) in the ANSWER BOOK, for example 1.6 E.
1.1 The process of adding impurities to intrinsic semiconductors is called a(n) ...
A intrinsic semiconductor.
B pure semiconductor.
C doping.
D purification. (2)
1.2 Consider the following structural formulae for compounds A and B.
These compounds have the same … and differ with …
A molecular formulae; positional isomers.
B molecular formulae; position of the functional group.
C molecules; positions.
D position of the functional group; structural formulae. (2)
1.3 Study the organic reaction below and answer the following question.
CH4 + Y → CO2 + H2O
Which ONE of the following does Y represent, and which is the correct reaction condition for products to form?
A H2O and excess water
B O2 and mild heat
C H2 and heat
D O2 and excess oxygen (2)
1.4 Which of the following sets is the correct for an N-type semiconductor?
1 | 2 | 3 | |
A | donor level | The extra electron is free to move | Not negatively charged |
B | acceptor band | Electrons in the valence band move from hole to hole | The absence of an electron creates the effect of a positive charge |
C | donor level | Electrons in the valence band move from hole to hole | Not negatively charged |
D | acceptor band | The extra electron is free to move | The absence of an electron creates the effect of a positive charge |
(2)
1.5 P-n junction
(i) In doping, a pure element is added to a semiconductor to improve the conductivity of the semiconductor.
(ii) In doping, a catalyst is added to a semiconductor to improve the conductivity of the semiconductor.
(iii) The n-region becomes positively charged because it has lost some electrons.
(iv) There is potential difference between the two sides of the diode.
(v) Electrons (few) gain enough thermal energy to cross the energy gap (from the valence band) to the conduction band.
Which ONE of the following combinations below is CORRECT for a p-n junction?
A (i) and (ii)
B (ii) and (iii)
C (i) and (iv)
D (iii) and (iv) (2) [10]
QUESTION 2 (Start on a new page.)
Consider the organic compounds represented by the letters A to H below and answer the questions that follow.
A | Hex-2-ene | E | 2-methylpropan-2-ol |
B | F | Ethylethanoate | |
C | G | ||
D | H | C5H12 |
2.1 Define the term functional group. (2)
2.2 Write down the letter(s) that represents the following:
2.2.1 A tertiary alcohol (1)
2.2.2 Unsaturated hydrocarbons (1)
2.2.3 An ester (1)
2.2.4 Hydrocarbons (1)
2.2.5 Positional isomers (1)
2.3 Write down the IUPAC name of the following:
2.3.1 D (1)
2.3.2 B (1)
2.3.3 G (1)
2.4 Write down the:
2.4.1 STRUCTURAL formula of compound F (2)
2.4.2 STRUCTURAL formula for the functional group of compound C (1)
2.4.3 MOLECULAR formula of compound A (1)
2.4.4 The name of the functional group of compound B (1)
2.4.5 STRUCTURAL formula of compound E (2) [17]
QUESTION 3 (Start on a new page.)
Students were observing the vapour pressure of three (3) organic compounds from a homologous series with a general formula CnH2n+2, represented by X, Y and Z. The number of carbon atoms of these organic compounds ranges between 3 carbon atoms and 5 carbon atoms. Their results were graphed as follows:
COMPOUND | VAPOUR PRESSURE (kPa) | MOLECULAR MASS (g.mol-1) |
X | 215 | 58 |
Y | 202 | 73 |
Z | 156 | 86 |
3.1 Define the term vapour pressure. (2)
3.2 Use the table above to draw a sketch graph of vapour pressure versus molecular mass. (3)
3.3 What hypothesis can be deduced from the graph? (1)
3.4 Give the industrial use of these organic compounds. (1)
3.5 Explain the difference in the vapour pressure of compound Y and Z. Refer to the MOLECULAR MASS, STRENGTH OF INTERMOLECULAR FORCES and THE ENERGY NEEDED. (3)
3.6 Which compound will have the …? (Write only X, Y or Z.)
3.6.1 highest viscosity (1)
3.6.2 lowest melting point (1)
3.6.3 highest boiling point (1) [13]
QUESTION 4 (Start on a new page.)
The table below shows the boiling points of four organic compounds, represented by the letters I to L, of comparable molecular mass.
COMPOUND | FORMULA | BOILING POINT (°C) | |
A | I | CH3OH | 80 |
B | J | CH2Cℓ2 | 40,1 |
C | K | CHCℓ3 | 61,8 |
D | L | CCℓ4 | 76,6 |
4.1 Define the term boiling point. (2)
4.2 In which homologous series does compound K in the table belong? (1)
4.3 Name the intermolecular forces in compound J. (1)
4.4 What trend can be observed from compound J to compound L, in the table? (1)
4.5 An investigation was conducted on the boiling points of compounds I and L.
4.5.1 Provide the IUPAC name of compound L. (1)
4.5.2 The comparison of I and L is a fair comparison. Give a reason why this is a true statement. (1)
4.5.3 Explain how the vapour pressure of compound I will compare to that of compound L. (2) [9]
QUESTION 5 (Start on a new page.)
Alcohol H can be converted to many other compounds and be a product of other reactions. As such alcohol H was used to form an organic compound called propyl butanoate. Study the table below and answer the questions that follow.
REACTION NUMBER | ORGANIC REACTION |
REACTION 1 | Alkene + R → Alcohol H |
REACTION 2 | Alkene + S → Alkane W |
REACTION 3 | Alcohol H + Br2 → Haloalkane + V |
REACTION 4 | Haloalkane + R → Alcohol H + T |
REACTION 5 | Alkane W + Y → Z + H2O |
5.1 Are the intermolecular forces in propyl butanoate WEAKER or STRONGER than those in alcohol H? Write only WEAKER or STRONGER. (1)
5.2 Identify alcohol H. (1)
5.3 Write down the type of reaction represented by the following reactions:
5.3.1 Reaction 1 (1)
5.3.2 Reaction 3 (1)
5.3.3 Reaction 5 (1)
5.4 For Reaction 2:
5.4.1 Write down the STRUCTURAL FORMULA for the alkene. (2)
5.4.2 Is compound S, ORGANIC or INORGANIC? (1)
5.4.3 Explain the answer to QUESTION 5.4.2 above. (1)
5.5 For Reaction 1, write down:
5.5.1 The balanced chemical equation using STRUCTURAL FORMULAE (3)
5.5.2 One reaction condition (1)
5.6 For Reaction 5, write down:
5.6.1 The STRUCTURAL FORMULA for alkane W (2)
5.6.2 NAME for compound Y (1)
5.6.3 FORMULA for compound Z (1) [17]
QUESTION 6 (Start on a new page.)
Study the diagram of a semiconductor below and answer questions that follow.
Note the following about semiconductors:
- Some semiconductors are formed by adding impurities to them and some formed at high temperatures where the atoms vibrate.
- Semiconductors are used in the manufacture of electronic devices such as diodes, transistors, and integrated circuits.
6.1 Which element represents a dopant in the diagram? Write only Si or B. (1)
6.2 How many valence electrons does this dopant have? (1)
6.3 What does A in the diagram above, represent? (1)
6.4 Define a semiconductor. (2)
6.5 Briefly explain what will happen if the semiconductor above is connected across the terminals of a cell. (2)
6.6 What is the purpose of doping? (1)
6.7 Identify the type of a semiconductor represented by the diagram above. (1) [9]
TOTAL: 75
DATA FOR TECHNICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS/TABEL 1: FISIESE KONSTANTES
NAAM/NAME | SIMBOOL/SYMBOL | WAARDE/VALUE |
Avogadro se konstante Avogadro’s constant | NA | 6,02 × 1023 mol-1 |
Molêre gaskonstante Molar gas constant | R | 8,31 J.K-1.mol-1 |
Standaarddruk Standard pressure | pθ | 1,013 × 105 Pa |
Molêre gasvolume teen STD Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standaardtemperatuur Standard temperature | Tθ | 273 K |
TABLE 2: FORMULAE/TABEL 2: FORMULES
n = m/M or/of n = N/NA or/of n = V / Vm | c = n/V or/of c = m / MV caVa na | pH= -log[H3O+] Kw = [H3O+][OH-] = 1x10-14 at /by 298K |
Eθcell = Eθcathode – Eθanode / Eθsel = Eθkatode – Eθanode | ||
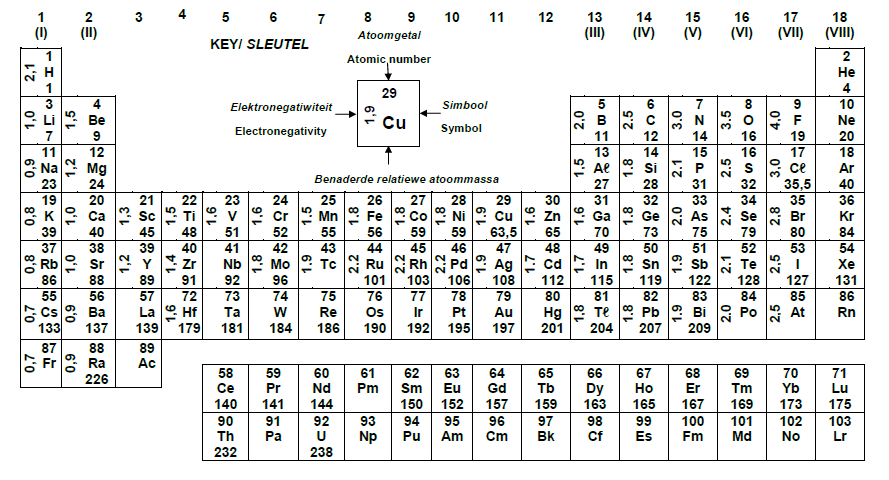
TABLE 3: THE PERIODIC TABLE OF ELEMENTS/TABEL 3: DIE PERIODIEKE TABEL VAN ELEMENTE
TABLE 4A: STANDARD REDUCTION POTENTIALS TABEL 4A: STANDAARD REDUKSIEPOTENSIALE
Half-reactions/Halfreaksies | Eθ(V) |
F2(g) + 2e− ⇌ 2F− Co3+ + e− ⇌ Co2+ H2O2 + 2H+ +2e− ⇌ 2H2O MnO−4+ 8H+ + 5e− ⇌ Mn2+ + 4H2O Cℓ2(g) + 2e− ⇌ 2Cℓ− Cr2O2−7+ 14H+ + 6e− ⇌ 2Cr3+ + 7H2O O2(g) + 4H+ + 4e− ⇌ 2H2O MnO2 + 4H+ + 2e− ⇌ Mn2+ + 2H2O Pt2+ + 2e− ⇌ Pt Br2(ℓ) + 2e− ⇌ 2Br− NO−3+ 4H+ + 3e− ⇌ NO(g) + 2H2O Hg2+ + 2e− ⇌ Hg(ℓ) Ag+ + e− ⇌ Ag NO−3+ 2H+ + e− ⇌ NO2(g) + H2O Fe3+ + e− ⇌ Fe2+ O2(g) + 2H+ + 2e− ⇌ H2O2 I2 + 2e− ⇌ 2I− Cu+ + e− ⇌ Cu SO2 + 4H+ + 4e− ⇌ S + 2H2O 2H2O + O2 + 4e− ⇌ 4OH− Cu2+ + 2e− ⇌ Cu SO2−4+ 4H+ + 2e− ⇌ SO2(g) + 2H2O Cu2+ + e− ⇌ Cu+ Sn4+ + 2e− ⇌ Sn2+ S + 2H+ + 2e− ⇌ H2S(g) 2H+ + 2e− ⇌ H2(g) Fe3+ + 3e− ⇌ Fe Pb2+ + 2e− ⇌ Pb Sn2+ + 2e− ⇌ Sn Ni2+ + 2e− ⇌ Ni Co2+ + 2e− ⇌ Co Cd2+ + 2e− ⇌ Cd Cr3+ + e− ⇌ Cr2+ Fe2+ + 2e− ⇌ Fe Cr3+ + 3e− ⇌ Cr Zn2+ + 2e− ⇌ Zn 2H2O + 2e− ⇌ H2(g) + 2OH− Cr2+ + 2e− ⇌ Cr Mn2+ + 2e− ⇌ Mn Aℓ3+ + 3e− ⇌ Aℓ Mg2+ + 2e− ⇌ Mg Na+ + e− ⇌ Na Ca2+ + 2e− ⇌ Ca Sr2+ + 2e− ⇌ Sr Ba2+ + 2e− ⇌ Ba Cs+ + e- ⇌ Cs K+ + e− ⇌ K Li+ + e− ⇌ Li | + 2,87 + 1,81 +1,77 + 1,51 + 1,36 + 1,33 + 1,23 + 1,23 + 1,20 + 1,07 + 0,96 + 0,85 + 0,80 + 0,80 + 0,77 + 0,68 + 0,54 + 0,52 + 0,45 + 0,40 + 0,34 + 0,17 + 0,16 + 0,15 + 0,14 0,00 − 0,06 − 0,13 − 0,14 − 0,27 − 0,28 − 0,40 − 0,41 − 0,44 − 0,74 − 0,76 − 0,83 − 0,91 − 1,18 − 1,66 − 2,36 − 2,71 − 2,87 − 2,89 − 2,90 - 2,92 − 2,93 − 3,05 |
TABLE 4B: STANDARD REDUCTION POTENTIALS TABEL 4B: STANDAARD REDUKSIEPOTENSIALE
Half-reactions/Halfreaksies | Eθ(V) |
Li+ + e− ⇌ Li K+ + e− ⇌ K Cs+ + e− ⇌ Cs Ba2+ + 2e−⇌ Ba Sr2+ + 2e− ⇌ Sr Ca2+ + 2e− ⇌ Ca Na+ + e− ⇌ Na Mg2+ + 2e− ⇌ Mg Aℓ3+ + 3e− ⇌ Aℓ Mn2+ + 2e−⇌ Mn Cr2+ + 2e− ⇌ Cr 2H2O + 2e− ⇌ H2(g) + 2OH− Zn2+ + 2e− ⇌ Zn Cr3+ + 3e− ⇌ Cr Fe2+ + 2e− ⇌ Fe Cr3+ + e− ⇌ Cr2+ Cd2+ + 2e− ⇌ Cd Co2+ + 2e− ⇌ Co Ni2+ + 2e− ⇌ Ni Sn2+ + 2e− ⇌ Sn Pb2+ + 2e− ⇌ Pb Fe3+ + 3e− ⇌ Fe 2H+ + 2e−⇌ H2(g) S + 2H+ + 2e− ⇌ H2S(g) Sn4+ + 2e− ⇌ Sn2+ Cu2+ + e−⇌ Cu+ SO2−4+ 4H+ + 2e− ⇌ SO2(g) + 2H2O Cu2+ + 2e− ⇌ Cu 2H2O + O2 + 4e− ⇌ 4OH− SO2 + 4H+ + 4e− ⇌ S + 2H2O Cu+ + e− ⇌ Cu I2 + 2e− ⇌ 2I− O2(g) + 2H+ + 2e−⇌ H2O2 Fe3+ +e−⇌ Fe2+ NO−3+ 2H+ + e− ⇌ NO2(g) + H2O Ag+ + e− ⇌ Ag Hg2+ + 2e− ⇌ Hg(ℓ) NO−3+ 4H+ + 3e− ⇌ NO(g) + 2H2O Br2(ℓ) + 2e− ⇌ 2Br− Pt2+ + 2 e− ⇌ Pt MnO2 + 4H+ + 2e−⇌ Mn2+ + 2H2O O2(g) + 4H+ + 4e− ⇌ 2H2O Cr2O2−7+ 14H+ + 6e− ⇌ 2Cr3+ + 7H2O Cℓ2(g) + 2e− ⇌ 2Cℓ− MnO−4+ 8H+ + 5e− ⇌ Mn2+ + 4H2O H2O2 + 2H+ +2e− ⇌ 2H2O Co3+ + e−⇌ Co2+ F2(g) + 2e− ⇌ 2F− | − 3,05 − 2,93 − 2,92 − 2,90 − 2,89 − 2,87 − 2,71 − 2,36 − 1,66 − 1,18 − 0,91 − 0,83 − 0,76 − 0,74 − 0,44 − 0,41 − 0,40 − 0,28 − 0,27 − 0,14 − 0,13 − 0,06 0,00 + 0,14 + 0,15 + 0,16 + 0,17 + 0,34 + 0,40 + 0,45 + 0,52 + 0,54 + 0,68 + 0,77 + 0,80 + 0,80 + 0,85 + 0,96 + 1,07 + 1,20 + 1,23 + 1,23 + 1,33 + 1,36 + 1,51 +1,77 + 1,81 + 2,87 |
MARKING GUIDELINE
QUESTION/VRAAG 1
1.1 C ✓✓ (2)
1.2 B ✓✓ (2)
1.3 D ✓✓ (2)
1.4 A ✓✓ (2)
1.5 D ✓✓ (2) [10]
QUESTION/VRAAG 2
2.1 An atom or a group of atoms that determine the chemistry of a molecule. ✓✓ OR An atom or a group of atoms that determine(s) the physical and chemical properties of a group of organic compounds.
2.2
2.2.1 E ✓ (1)
2.2.2 A and/en D ✓ (1)
2.2.3 F ✓ (1)
2.2.4 A, D and/en H ✓ (1)
2.2.5 A and/en D ✓ (1)
2.3
2.3.1 pent-1-ene ✓ (accept 1-pentene/aanvaar 1-penteen) pent-1-een (1)
2.3.2 Propanone / Propanoon ✓ (1)
2.3.3 Hexanoic Acid / Heksanoësuur ✓ (1)
2.4
2.4.1
MARKING CRITERIA:
NOTE: If a bond or hydrogen is missing ½ NASIENKRITERIA:
LET WEL: Indien ʼn binding of waterstof ontbreek ½. |
2.4.2 ✓ (1)
2.4.3 C5H11 ✓ (1)
2.4.4 Carboxyl group / Karboksiel-groep ✓ (1)
2.4.5 ✓✓
MARKING CRITERIA:
NOTE: If a bond or hydrogen is missing ½ NASIENKRITERIA:
LET WEL: Indien ʼn binding of waterstof ontbreek ½ (2) [17] |
QUESTION/VRAAG 3
3.1 The pressure exerted by a vapour at equilibrium with its liquid phases of a substances are at equilibrium. ✓✓/ Die druk uitgeoefen deur ʼn damp in ewewig met sy vloeistof in ʼn geslote sisteem. (2)
3.2
Marking criteria
1 mark | Both axes correctly labelled ✓ |
1 mark | All points indicated ✓ |
1 mark | Shape of the graph ✓ |
(3)
3.3 The greater the molecular mass of organic compounds/alkanes, the lower the vapour pressure. ✓/ Hoe groter die molekulêre massa van organiese verbindings/alkane, hoe laer is die dampdruk. (1) 3.4 Used as fuels/Word as brandstof gebruik ✓ (1)
3.5
- Compound Z / pentane has 5 carbon atoms which makes it to have a longer chain / greater molecular mass than compound Y / butane which has 4 carbon atoms which makes it to have a shorter chain/less molecular mass than compound Z. ✓
Verbinding Z / pentaan het 5 koolstofatome wat maak dat dit ʼn langer ketting / groter molekulêre massa as verbinding Y het / butaan wat 4 koolstofatome het, wat maak dat dit ʼn korter ketting / minder molekulêre massa as verbinding Z het. - The greater the molecular mass / longer the chain length the stronger the Intermolecular forces / London forces in compound Z are stronger than those in compound Y which are weaker. ✓
Hoe groter die molekulêre massa / langer die kettingslengte, hoe sterker is die intermolekulêre kragte / Londonkragte in verbinding Z is sterker as die in verbinding Y, wat swakker is. - More energy will be required to overcome intermolecular forces/London forces in Compound Z than in compound Y where less energy will be required to overcome intermolecular forces. ✓
Meer energie sal benodig word om intermolekulêre kragte/Londenkragte in verbinding Z te oorkom as in verbinding Y waar minder energie nodig sal wees om intermolekulêre kragte te oorkom. - (Compound Z / pentane will have lower vapour pressure than compound Y/butane which will have higher vapour pressure than Compound Z.) • (Verbinding Z / pentaan sal laer dampdruk hê as verbinding Y / butaan wat hoër dampdruk as verbinding Z sal hê.)
OR/OF
- Compound Y / Butane has 4 carbon atoms which makes it to have a shorter chain / lesser molecular mass than compound Z / pentane which has 5 carbon atoms which makes it to have a longer chain / greater molecular mass than compound Y.
Verbinding Y / Butaan het 4 koolstofatome wat maak dat dit ʼn korter ketting / minder molekulêre massa het as verbinding Z / pentaan wat 5 koolstofatome het wat maak dat dit ʼn langer ketting / groter molekulêre massa as verbinding Y het. - The lesser the molecular mass / shorter the chain the weaker the intermolecular forces/London forces in compound Y than in Compound Z which are stronger.
- Hoe kleiner die molekulêre massa / korter die ketting hoe swakker is die intermolekulêre kragte/Londen kragte in verbinding Y as in verbinding Z wat sterker is.
- Less energy will be required to overcome intermolecular forces/London forces in compound Y than in compound Z where more energy will be required to overcome intermolecular forces.
Minder energie sal benodig word om intermolekulêre kragte/Londen kragte in verbinding Y te oorkom as in verbinding Z waar meer energie benodig sal word om intermolekulêre kragte te oorkom. - (Compound Y / butane will have higher vapour pressure than compound Z / pentane which will have lower vapour pressure than Compound Y.) (Verbinding Y / butaan sal hoër dampdruk hê as verbinding Z / pentaan wat laer dampdruk as verbinding Y sal hê.) (3)
3.6
3.6.1 Z ✓ (1)
3.6.2 X ✓ (1)
3.6.3 X ✓ (1) [13]
QUESTION/VRAAG 4
4.1 The temperature at which the vapour pressure is equal to the atmospheric pressure. ✓✓ Die temperatuur waarby die dampdruk gelyk aan die atmosferiese druk is. (2)
4.2 Haloalkanes/alkyl halides/Haloalkane/alkielhaliede ✓ (1)
4.3 (London forces and) Dipole-dipole intermolecular forces ✓/ (Londenkragte en) Dipool-dipool intermolekulêre kragte (1)
4.4 The boiling point increase with an increase in the number of chlorine atoms in haloalkanes. ✓/ Die kookpunt verhoog met ʼn toename in die aantal chlooratome in haloalkane. (1)
4.5
4.5.1 Tetrachloromethane / Tetrachlorometaan ✓ (1)
4.5.2 One independent variable ✓
Accept same chain length / same number of carbons
4.5.3 The vapour pressure of compound I will be lower than the vapour pressure of compound L. ✓✓
OR
The vapour pressure of compound L will be higher than the vapour pressure of compound I.
QUESTION/VRAAG 5
5.1 Weaker/Swakker ✓ (1)
5.2 Propanol ✓ (1)
5.3
5.3.1 Addition reaction/hydration ✓
5.3.2 Substitution reaction/halogenation/bromination ✓
5.3.3 Combustion/Oxidation ✓/
5.4
5.4.1 ✓
| MARKING CRITERIA :
NOTE: If a bond or hydrogen is missing ½ |
5.4.2 Inorganic/Anorganies ✓ (1)
5.4.3 It does not have carbon as the main element. ✓/
5.5
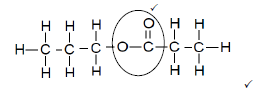
5.5.1
MARKING CRITERIA
NOTE: Accept molecular structure of H2O |
5.5.2 Excess water /H2O in concentration H2SO4 / Diluted sulphuric acid / H2SO4 ✓
5.6
5.6.1 ✓ ✓
MARKING CRITERIA:
NOTE: If a bond or hydrogen is missing ½ |
5.6.2 Oxygen (gas) / Suurstof (gas) ✓ (1)
5.6.3 CO2 ✓ (1) [17]
QUESTION/VRAAG 6
6.1 Boron / B ✓ (1)
6.2 Three / Drie (3) ✓ (1)
6.3 Free holes that are positively charged/positive holes. ✓
6.4 Semiconductor is a material that has electrical conductivity between that of a conductor and an insulator. ✓✓
6.5 If the semiconductor is connected across the terminals of a cell, the electrons in the valence band move from hole to hole. ✓ The absence of an electron creates the effect of a positive charge. ✓
6.6 Improves conductivity of the semiconductor ✓
6.7 P-type / P-tipe ✓ (1) [9]
TOTAL: 75