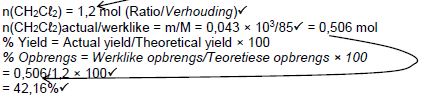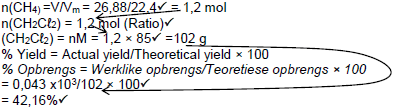PHYSICAL SCIENCES PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS SEPTEMBER 2016
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES P2
GRADE 12
MEMORANDUM
NATIONAL SENIOR CERTIFICATE
SEPTEMBER 2016
GUIDELINES FOR MARKING
This section provides guidelines for the way in which marks will be allocated. The broad principles must be adhered to in the marking of Physical Sciences tests and examinations.
1.1MARK ALLOCATION
1.1.1 Definitions/: Two marks will be awarded for a correct definition. No marks will be awarded for an incorrect or partially correct definition.
1.1.2 Calculations:
- Marks will awarded for: correct formula, correct substitution, correct answer with unit.
- No marks will be awarded if an incorrect or inappropriate formula is used, even though there may be relevant symbols and applicable substitutions.
1.1.3 Explanations and interpretations
Allocation of marks to questions requiring interpretation or explanation e.g. AS 1.4, 2.2, 2.3, 3.1, 3.2 and 3.3, will differ and may include the use of rubrics, checklists, memoranda, etc. In all such answers emphasis must be placed on scientific concepts relating to the question.
1.2 FORMULAE AND SUBSTITUTIONS
1.2.1 Mathematical manipulations and change of subjects of appropriate formulae carry no marks, but if a candidate starts with the correct formula and then changes the subject of the formula incorrectly, marks will be awarded for the formula and the correct substitutions. The mark for the incorrect numerical answer is forfeited.
1.2.2 When an error is made during substitution into a correct formula, a mark will be awarded for the correct formula and for the correct substitutions, but no further marks will be given.
1.2.3 Marks are only awarded for a formula if a calculation had been attempted, i.e. substitutions have been made or a numerical answer given.
1.2.4 Marks can only be allocated for substitutions when values are substituted into formulae and not when listed before a calculation starts.
1.2.5 All calculations, when not specified in the question, must be done to two decimal places.
1.3 UNITS
1.3.1 Candidates will only be penalised once for the repeated use of an incorrect unit within a question or sub-question.
1.3.2 Units are only required in the final answer to a calculation.
1.3.3 Marks are only awarded for an answer, and not for a unit per se. Candidates will therefore forfeit the mark allocated for the answer in each of the following situations:
- correct answer + wrong unit
- wrong answer + correct unit
- correct answer + no unit.
1.3.4 SI units must be used except in certain cases, e.g. V.m-1 instead of N.C-1, and cm.s-1 or km.h-1 instead of m.s-1 where the question warrants this. (This instruction only applies to Paper 1).
1.4 POSTIVE MARKING
Positive marking regarding calculations will be followed in the following cases:
1.4.1 Sub-question to sub-question: When a certain variable is calculated in one sub-question (e.g. 3.1) and needs to be substituted in another (3.2 or 3.3), e.g. if the answer for 3.1 is incorrect and is substituted correctly in 3.2 or 3.3, full marks are to be awarded for the subsequent sub-questions.
1.4.2 A multi-step question in a sub-question: If the candidate has to calculate, for example, current in the first step and gets it wrong due to a substitution error, the mark for the substitution and the final answer will be forfeited.
1.4.3 If a final answer to a calculation is correct, full marks will not automatically be awarded. Markers will always ensure that the correct/ appropriate formula is used and that workings, including substitutions, are correct.
1.4.4 Questions where a series of calculations have to be made (e.g. a circuit diagram question) do not necessarily always have to follow the same order. FULL MARKS will be awarded provided it is a valid solution to the problem. However, any calculation that will not bring the candidate closer to the answer than the original data, will not count any marks.
1.4.5 If one answer or calculation is required, but two given by the candidate, only the first one will be marked, irrespective of which one is correct. If two answers are required, only the first two will be marked, etc.
1.4.6 Normally, if based on a conceptual mistake, an incorrect answer cannot be correctly motivated. If the candidate is therefore required to motivate in question 3.2 the answer given to question 3.1, and 3.1 is incorrect, no marks can be awarded for question 3.2. However, if the answer for e.g. 3.1 is based on a calculation, the motivation for the incorrect answer for 3.2 could be considered.
1.4.7 If instructions regarding method of answering are not followed, e.g. the candidate does a calculation when the instruction was to solve by construction and measurement, a candidate may forfeit all the marks for the specific question.
1.4.8 For an error of principle, no marks are awarded (Rule 1) e.g. If the potential difference is 200 V and resistance is 25 Ω, calculate the current.
CORRECT ANSWER (1) POSSIBLE ANSWER (2) POSSIBLE
I = V ✓ R = V ✓ R = V ✓ R = V ✓ I = V✓
R I I I R
= 200 ✓ = 200 ✓ = 200 ✓ I = R ✓ = 8A✓
25 25 25 V
=8A ✓ =8A =8A = 25
200
= 0.125A
1.5 GENERAL PRINCIPLES OF MARKING IN CHEMISTRY
The following are a number of guidelines that specifically apply to Paper 2.
1.5.1 When a chemical FORMULA is asked, and the NAME is given as answer, only one of the two marks will be awarded. The same rule applies when the NAME is asked and the FORMULA is given.
1.5.2 When redox half-reactions are to be written, the correct arrow should be used. If the equation
H2S → S + 2H+ + 2e- (2/2)
is the correct answer, the following marks will be given:
H2S ⇋ S + 2H+ + 2e- (1/2)
H2S ← S + 2H+ + 2e- (0/2)
S + 2H+ + 2e- ← H2S (2/2)
S + 2H+ + 2e- ⇋ H2S (0/2)
1.5.3 When candidates are required to give an explanation involving the relative strength of oxidising and reducing agents, the following is unacceptable:
- Stating the position of a substance on Table 4 only (e.g. Cu is above Mg).
- Using relative reactivity only (e.g. Mg is more reactive than Cu).
- The correct answer would for instance be: Mg is a stronger reducing agent than Cu, and therefore Mg will be able to reduce Cu2+ ions to Cu. The answer can also be given in terms of the relative strength as electron acceptors and donors.
1.5.4 One mark will be forfeited when the charge of an ion is omitted per equation.
1.5.5 The error carrying principle does not apply to chemical equations or half-reactions. For example, if a learner writes the wrong oxidation/reduction half-reaction in the sub-question and carries the answer to another sub-question (balancing of equations or calculations of Eθcell) then the learner is not credited for this substitution.
1.5.6 When a calculation of the cell potential of a galvanic cell is expected, marks will only be awarded for the formula if one of the formulae indicated on the data sheet (Table 2) is used. The use of any other formula using abbreviations etc. will carry no marks.
1.5.7 In the structural formula of an organic molecule all hydrogen atoms must be shown. Marks will be deducted if hydrogen atoms are omitted.
1.5.8 When a structural formula is asked, marks will be deducted if the candidate writes the condensed formula.
1.5.9 When an IUPAC name is asked, and the candidate omits the hyphen (e.g. instead of 1-pentene the candidate writes 1 pentene), marks will be forfeited.
QUESTION 1
1.1 B ✓✓ (2)
1.2 C ✓✓ (2)
1.3 A ✓✓ (2)
1.4 C ✓✓ (2)
1.5 C ✓✓ (2)
1.6 A ✓✓ (2)
1.7 A ✓✓ (2)
1.8 C ✓✓ (2)
1.9 B ✓✓ (2)
1.10 D ✓✓ (2)
[20]
QUESTION 2
2.1
2.1.1 D ✓ or F (1)
2.1.2 E ✓ (1)
2.2
2.2.1 2,4-dimethyl ✓hex-1-ene ✓
Accept: 2,4-dimethyl-1-hexene
Marking criteria
|
2.2.2
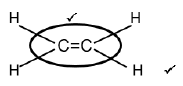 | Marking criteria:
|
(2)
2.3
2.3.1 CnH2n + 2 ✓ (1)
2.3.2 CO2 ✓ and H2O ✓ (2)
2.4
2.4.1
| 2-chloro✓propane✓ | IF 2 chloropropane(1/2) |
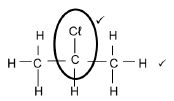 | Marking criteria
|
| Notes: Condensed structural formula or semi structural formule. 1/2 One or more H atoms omitted. 1/2 |
(2)
(2)(4)
2.4.2 Positional✓ (1)
2.5
| OPTION1 % O = 32 / M × 100 = 12,5 ✓ M = 256 g∙mol-1 n(12) + 1(2n) + 32 = 256 ✓ or/of n(12) + 1(2n) = 224 n = 16 % C = 16(12)/256 × 100 ✓ = 75 % X = 75✓ |
| OPTION 2 % ( H and C) = 87,5% ✓ (100% - 12,5%) RATIO H : C 2n × 1 : n × 12 1 : 6 ✓ %C = 6/7 × 87,5%✓ = 75%✓ |
(4)
[18]
QUESTION 3
3.1
3.1.1
- Substitution ✓
OR
Hydrolysis ✓ (1) - 2-butanol✓✓
| Marking criteria: Stem i.e. butanol. 1/2 Whole name correct. 2/2 |
(2)
3.1.2 Reaction 2✓ (1)
3.1.3
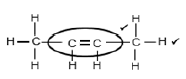
Marking criteria
|
(2)
3.2
3.2.1 To avoid fire./Alcohol catching flame/Alcohol is flammable✓ (1)
3.2.2
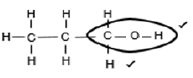
Marking criteria:
|
(2)
3.2.3 Methanoic acid✓ (1)
[10]
QUESTION 4
4.1 Compounds with the same molecular formula ✓ but different length of (carbon) chains.✓ (2)
4.2 50✓ (kPa) (1)
4.3
Marking criteria
Notes
|
(2)
4.4
- Chain in A longer than that of in B/surface area of A larger than that of B/A is less spherical than B. ✓
- Strength of London forces✓/induced dipole/dispersion forces STRONGER in A ✓ than in B.
OR
- Chain in B shorter than that in A/surface area of B smaller than that of A/B is more spherical than A.✓
- Strength of London forces✓/induced dipole/dispersion forces WEAKER in B ✓ than in A. (3)
4.5
4.5.1 London forces/induced dipole forces/dispersion forces.✓ (2)
4.5.2 Between molecules of D there are hydrogen bonds ✓ in addition to dipole-dipole forces and London forces/dispersion forces/induced dipole forces.
Between molecules of F there are dipole dipole forces ✓ in addition to London forces/dispersion forces/induced dipole forces.
Hydrogen bonds in D are stronger ✓ than dipole-dipole forces in F.
OR
Dipole-dipole forces in F are weaker than hydrogen bonds in D. (3)
4.5.3 Substitution/Halogenation/Chlorination (1)
4.5.4
| Marking criteria *Divide by 22,4 *Use of ratio. *Divide or multiply by 85 *% yield *Final answer✓ |
OPTION 1
Accepted range: 42,16 to 42,5% |
OPTION 2
|
| OPTION 3 V(CH2Cℓ2) = 26,88dm3 (Ratio)✓  Accepted range: 42,16 to/tot 42,5% |
(5)
[19]
QUESTION 5
5.1 Exothermic ✓ ΔT is positive. ✓
OR
T final is higher ✓
OR
T initial is lower ✓ (2)
5.2 Nature of reacting substances. ✓ (1)
5.3 EQUAL TO ✓ Same amount of metal used. ✓ (2)
5.4
5.4.1 Experiment 2✓(1)
5.4.2 Mg is a stronger reducing agent ✓ than Zn. ✓(2)
5.5 Increase in temperature increases kinetic energy of particles. ✓
More particles will have sufficient/enough kinetic energy./Ea.≥Ek. ✓
More effective collisions per unit time/second. (Frequency of effective collisions increases). ✓ (3)
[11]
QUESTION 6
6.1 Homogeneous ✓
Reactants and products are in the same phase. ✓(2)
6.2
6.2.1
- More N2 added/Increase ✓in [N2] ✓ (1)
- Pressure ✓ decreases ✓ (by increase in volume) (1)
6.2.2 Equal to✓ (1)
6.3
6.3.1
Marking Criteria n(H2) change = n(H2) initial - n(H2) equilibrium. ✓ USE RATIO for n(N2) change and n (NH3) change. ✓ n(equilibrium) = n(initial) - n (change) for both n(N2) and n(NH3) ✓ Divide n(equilibrium) by 1 to calculate c(equilibrium). ✓ Correct Kc expression (formulae in square brackets). ✓ Substitution of Kc value of 1,426 × 103✓ Substitution of concentrations into Kc expression. ✓ Calculate n. ✓ Substitute value for n and 28 for M in n = m/M ✓ Final answer ✓ |
OPTION 1
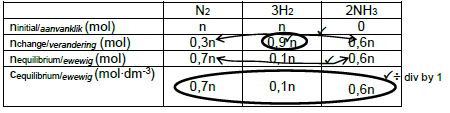
OPTION 2: Concentrations in mol∙dm-3
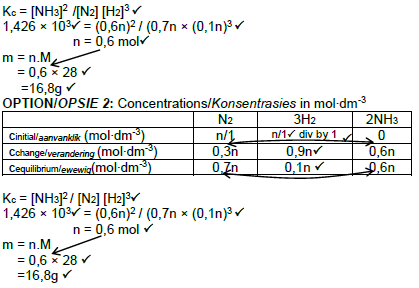
(10)
6.3.2 Kc Decreases ✓
Increase in temperature favours the endothermic reaction. ✓
Reverse reaction is favoured ✓ (decreasing [NH3], increasing [N2] and [H2]). (3)
[18]
QUESTION 7
7.1 Solution with known concentration. ✓✓ (2)
7.2 Improve accuracy of results./Ensuring more accurate results. ✓ (1)
7.3 n = cV ✓
= 0,02 × 25 × 10-3 ✓
= 5 × 10-4 mol ✓ (0,0005 mol) (3)
7.4 POSITIVE MARKING FROM QUESTION 7.3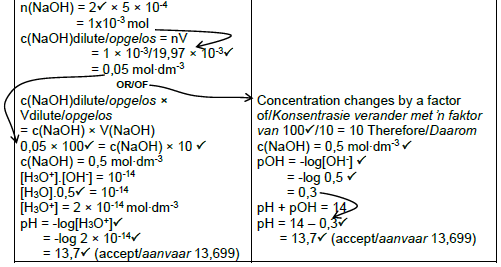
Marking Criteria
- Use mole ratio/Gebruik mol verhouding: n(H2C2O4) : n(NaOH) = 1: 2 ✓
- Substitute volume and number of moles to calculate c(NaOH)dilute. ✓
- c(NaOH)dilute × 100 = c(NaOH) × 10 to calculate c(NaOH) before dilution.✓
- Formule of pH. ✓
- Subsitute c(NaOH)concentrated in [H3O+].[OH-] = 10-14 ✓
- Substitute value for [H3O+] in formula for pH.✓
- Final answer:13,7 ✓ (accept 13,699)
Notes:
Wrong formula for pH e.g pH = -log[OH-]; pOH = -log[NaOH]
No marks for substitution and answer in the pH calculation part. (8)
[14]
QUESTION 8
8.1
8.1.1 Provides path for movement of ions. ✓ (1)
8.1.2 0 ✓ (V) (1)
8.1.3 Platinum or ✓ (1)
8.1.4 Iron(III) ion or Fe3+ ✓ (1)
8.1.5 Fe3+ + e- ✓→ Fe2+✓ (2)
8.2 E0cell/sel = E0cathode – E0anode ✓
0,83✓= 0,77√- E0anode
E0anode = -0,06 ✓ (V)
X is Fe ✓ (Iron) (5)
Notes
|
8.3 Fe / Fe3+ // Fe2+, Fe3+ / Pt Accept: X / X3+ // Fe3+, Fe2+ / Pt (3)✓✓✓
[14]
QUESTION 9
9.1
9.1.1
Electrolytic ✓
Electrical energy to chemical energy. ✓(2)
9.1.2 2H2O + 2e- → H2 + 2OH-✓✓
Ignore phases
| Notes 2H2O + 2e- ⇋ H2 + 2OH- (1/2) H2 + 2OH- ← 2H2O + 2e- (2/2) H2 + 2OH- ⇋ 2H2O + 2e- (0/2) 2H2O + 2e- ← H2 + 2OH- (0/2) |
(2)
9.1.3 Cu or Copper ✓ (1)
9.1.4 Decreases ✓
Cu2+ (or Copper(II) ions) are reduced✓ to Cu ✓ (or Copper).(3)
9.2
9.2.1 Set ions free to move.✓ (1)
9.2.2 Na+ is a weaker oxidising agent✓ than Al3+ ✓ Al3+are reduced(to Al). ✓
OR
Al3+ is a stronger oxidising agent ✓ than Na+ ✓. Al3+ are reduced (to Al) ✓ (3)
[12]
QUESTION 10
10.1
10.1.1 Nitrogen✓ (1)
10.1.2 Iron ✓ or Iron oxides (1)
10.2 n(NO) = m/M= 1,12/28 ✓= 0,04 mol
1 mol NO : 1 mol NO2
n(NO2) = 0,04 mol ✓
ΔH = -149,1 kJ for every/vir elke 2mol NO
= -149,1 kJ/2mol NO × 0,04 mol NO ✓
= -2,98 kJ ✓
Marking Criteria
|
(4)
10.3
10.3.1 PROTOLYTIC✓ (1)
10.3.2 NH3 + HNO3 ✓ → NH4NO3 ✓ Bal. ✓
Notes:
|
(3)
10.4 m(N) = 3/11 ✓ × 60/100 × 50 ✓ = 8,18 kg ✓
Marking Criteria
|
(3)
10.5 Eutrophication ✓ OR Dead zones ✓ (1)
[14]
TOTAL: 150