PHYSICAL SCIENCES (CHEMISTRY) PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS NOVEMBER 2016
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES (CHEMISTRY)
PAPER TWO (P2)
GRADE 12
NSC EXAM PAPERS AND MEMOS
NOVEMBER 2016
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Write down the question number (1.1–1.10), choose the answer and make a cross (X) over the letter (A–D) of your choice in the ANSWER BOOK.
EXAMPLE:
1.11 ![]()
1.1 In a chemical reaction an oxidising agent will …
- lose protons.
- gain protons.
- lose electrons.
- gain electrons. (2)
1.2 A catalyst is added to a reaction mixture at equilibrium.
Which ONE of the following statements about the effect of the catalyst is FALSE?
- The rate of the forward reaction increases.
- The rate of the reverse reaction increases.
- The equilibrium position shifts to the right.
- The equilibrium position remains unchanged. (2)
1.3 What product will be formed when an alkene reacts with water vapour (H2O) in the presence of an acid catalyst?
- Ester
- Alkane
- Alcohol
- Aldehyde (2)
1.4 Which ONE of the following represents a SUBSTITUTION REACTION?
- CH2 = CH2 + HBr → CH3CH2Br
- CH2 = CH2 + H2O → CH3CH2OH
- CH3CH2OH → CH2 = CH2 + H2O
- CH3CH2OH + HBr → CH3CH2Br + H2O (2)
1.5 Consider the two organic molecules I and II below. 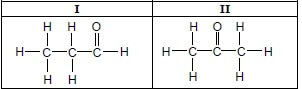
Which ONE of the following represents the homologous series to which compound I and compound II belong? (2)
I | II | |
A | Ketones | Alcohols |
B | Aldehydes | Ketones |
C | Aldehydes | Alcohols |
D | Ketones | Aldehydes |
1.6 Consider the balanced equations for three reactions represented below:
- N2(g) + 3H2(g) ⇌ 2NH3(g)
- 4NH3(g) + 5O2(g) ⇌ 4NO(g) + 6H2O(g)
- 2NO(g) + O2(g) ⇌ 2NO2(g)
Which of the above reactions form(s) part of the Ostwald process?
- I only
- II only
- III only
- II and III only (2)
1.7 Which ONE of the following pairs is NOT a conjugate acid-base pair?
- H3O+ and OH−
- NH4+ and NH3
- H2PO4- and HPO42-
- H2CO3 and HCO3- (2)
1.8 The reaction between hydrogen gas and iodine gas reaches equilibrium in a closed container according to the following balanced equation:
H2(g) + I2(g) ⇌ 2HI(g)
Which ONE of the graphs below shows the relationship between the amount of HI(g) at equilibrium and the pressure in the container at constant temperature? (2) 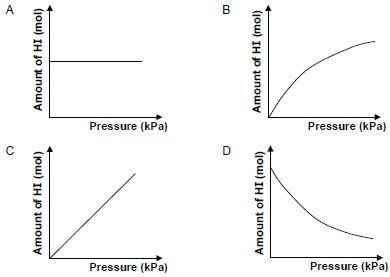
1.9 Which ONE of the equations below represents the half-reaction occurring at the CATHODE of an electrochemical cell that is used to electroplate an object?
- Ag → Ag+ + e-
- Cr3+ + 3e- → Cr
- Cr3+ + e- → Cr2+
- Cu2+ + e- → Cu+ (2)
1.10 Equal amounts of magnesium (Mg) powder react respectively with equal volumes and equal concentrations of HCℓ(aq) and H2SO4(aq), as shown below. 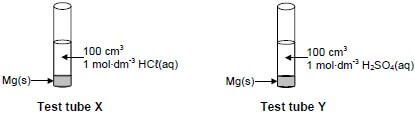
The magnesium is in EXCESS.
Consider the following statements regarding these two reactions:
- The initial rate of the reaction in test tube X equals the initial rate of the reaction in test tube Y.
- After completion of the reactions, the mass of magnesium that remains in test tube X will be greater than that in test tube Y.
- The amount of hydrogen gas formed in X is equal to the amount of hydrogen gas formed in Y.
Which of the above statements is/are TRUE?
- I only
- II only
- III only
- I and III only (2)
[20]
QUESTION 2 (Start on a new page.)
The letters A to F in the table below represent six organic compounds. 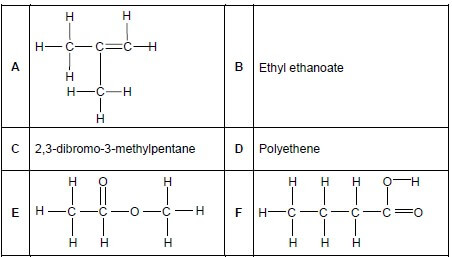
2.1 Write down the LETTER that represents the following:
2.1.1 A hydrocarbon (1)
2.1.2 A functional isomer of compound F (1)
2.1.3 A compound which belongs to the same homologous series as compound B (1)
2.1.4 A plastic (1)
2.2 Write down the STRUCTURAL FORMULA of EACH of the following:
2.2.1 Compound C (3)
2.2.2 The acid used to prepare compound B (2)
2.2.3 The monomer used to make compound D (2)
2.3 Compound A reacts with an unknown reactant, X, to form 2-methylpropane. Write down the:
2.3.1 NAME of reactant X (1)
2.3.2 Type of reaction that takes place (1)
[13]
QUESTION 3 (Start on a new page.)
The boiling points of three isomers are given in the table below.
ISOMERS | BOILING POINT (°C) | |
A | 2,2-dimethylpropane | 9 |
B | 2-methylbutane | 28 |
C | pentane | 36 |
3.1 Define the term structural isomer. (2)
3.2 What type of isomers (POSITIONAL, CHAIN or FUNCTIONAL) are these three compounds? (1)
3.3 Explain the trend in the boiling points from compound A to compound C. (3)
3.4 Which ONE of the three compounds (A, B or C) has the highest vapour pressure? Refer to the data in the table to give a reason for the answer. (2)
3.5 Use MOLECULAR FORMULAE and write down a balanced equation for the complete combustion of compound B. (3)
[11]
QUESTION 4 (Start on a new page.)
Butane (C4H10) is produced in industry by the THERMAL cracking of long-chain hydrocarbon molecules, as shown in the equation below. X represents an organic compound that is produced.
C10H22 → X + C4H10
4.1 Write down:
4.1.1 ONE condition required for THERMAL cracking to take place (1)
4.1.2 The molecular formula of compound X (1)
4.1.3 The homologous series to which compound X belongs (1)
4.2 A mixture of the two gases, compound X and butane, is bubbled through bromine water, Br2(aq), in a conical flask, as illustrated below. THE REACTION IS CARRIED OUT IN A DARKENED ROOM. 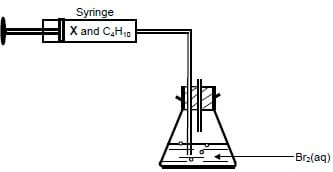
The colour of the bromine water changes from reddish brown to colourless when the mixture of the two gases is bubbled through it.
Which ONE of the gases (X or BUTANE) decolorises the bromine water? Explain the answer. (4)
4.3 Study the flow diagram below, which represents various organic reactions, and answer the questions that follow. 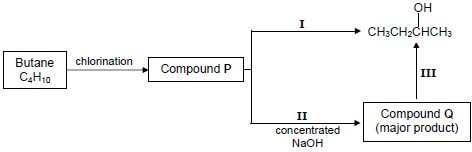
Write down the:
4.3.1 IUPAC name of compound P (2)
4.3.2 Type of reaction labelled I (1)
4.3.3 Structural formula of compound Q (2)
4.3.4 The type of addition reaction represented by reaction III (1)
[13]
QUESTION 5 (Start on a new page.)
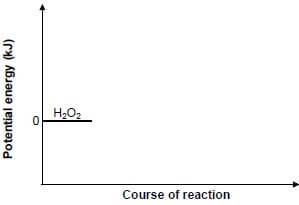
Hydrogen peroxide, H2O2, decomposes to produce water and oxygen according to the following balanced equation:
2H2O2(ℓ) → 2H2O(ℓ) + O2(g)
5.1 The activation energy (EA) for this reaction is 75 kJ and the heat of reaction (ΔH) is –196 kJ.
5.1.1 Define the term activation energy. (2)
5.1.2 Redraw the set of axes below in your ANSWER BOOK and then complete the potential energy diagram for this reaction.
Indicate the value of the potential energy of the following on the y-axis:
- Activated complex
- Products (3)
(The graph does NOT have to be drawn to scale.)
When powdered manganese dioxide is added to the reaction mixture, the rate of the reaction increases.
5.1.3 On the graph drawn for QUESTION 5.1.2, use broken lines to show the path of the reaction when the manganese dioxide is added. (2)
5.1.4 Use the collision theory to explain how manganese dioxide influences the rate of decomposition of hydrogen peroxide. (3)
5.2 Graphs A and B below were obtained for the volume of oxygen produced over time under different conditions.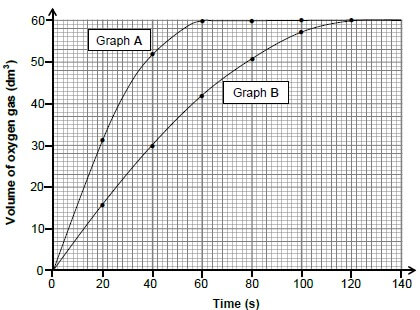
5.2.1 Calculate the average rate of the reaction (in dm3∙s-1) between t = 10 s and t = 40 s for graph A. (3)
5.2.2 Use the information in graph A to calculate the mass of hydrogen peroxide used in the reaction. Assume that all the hydrogen peroxide decomposed. Use 24 dm3·mol-1 as the molar volume of oxygen. (4)
5.2.3 How does the mass of hydrogen peroxide used to obtain graph B compare to that used to obtain graph A? Choose from GREATER THAN, SMALLER THAN or EQUAL TO. (1)
5.3 Three energy distribution curves for the oxygen gas produced under different conditions are shown in the graph below.
The curve with the solid line represents 1 mol of oxygen gas at 90 °C. 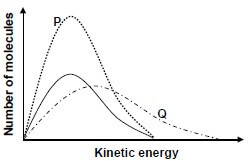
Choose the curve (P or Q) that best represents EACH of the following situations:
5.3.1 1 mol of oxygen gas produced at 120 °C (1)
5.3.2 2 moles of oxygen gas produced at 90 °C (1)
[20]
QUESTION 6 (Start on a new page.)
Hydrogen gas, H2(g), reacts with sulphur powder, S(s), according to the following balanced equation:
H2(g) + S(s) ⇌ H2S(g) ∆H < 0
The system reaches equilibrium at 90 °C.
6.1 Define the term chemical equilibrium. (2)
6.2 How will EACH of the following changes affect the number of moles of H2S(g) at equilibrium?
Choose from INCREASES, DECREASES or REMAINS THE SAME.
6.2.1 The addition of more sulphur (1)
6.2.2 An increase in temperature
Use Le Chatelier's principle to explain the answer. (4)
6.3 The sketch graph below was obtained for the equilibrium mixture. 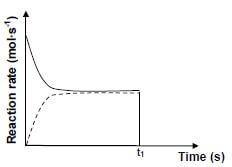
A catalyst is added to the equilibrium mixture at time t1.
Redraw the graph above in your ANSWER BOOK. On the same set of axes, complete the graph showing the effect of the catalyst on the reaction rates. (2)
Initially 0,16 mol H2(g) and excess S(s) are sealed in a 2 dm3 container and the system is allowed to reach equilibrium at 90 °C.
An exact amount of Pb(NO3)2 solution is now added to the container so that ALL the H2S(g) present in the container at EQUILIBRIUM is converted to PbS(s) according to the following balanced equation:
Pb(NO3)2(aq) + H2S(g) → PbS(s) + 2HNO3(aq)
The mass of the PbS precipitate is 2,39 g.
6.4 Calculate the equilibrium constant Kc for the reaction H2(g) + S(s) ⇌ H2S(g) at 90 °C. (9)
[18]
QUESTION 7 (Start on a new page.)
7.1 A learner dissolves ammonium chloride (NH4Cℓ) crystals in water and measures the pH of the solution.
7.1.1 Define the term hydrolysis of a salt. (2)
7.1.2 Will the pH of the solution be GREATER THAN, SMALLER THAN or EQUAL TO 7? Write a relevant equation to support your answer. (3)
7.2 A sulphuric acid solution is prepared by dissolving 7,35 g of H2SO4(ℓ) in 500 cm3 of water.
7.2.1 Calculate the number of moles of H2SO4 present in this solution. (2) Sodium hydroxide (NaOH) pellets are added to the 500 cm3 H2SO4 solution. The balanced equation for the reaction is:
H2SO4(aq) + 2NaOH(s) → Na2SO4(aq) + 2H2O(ℓ)
After completion of the reaction, the pH of the solution was found to be 1,3. Assume complete ionisation of H2SO4.
7.2.2 Calculate the mass of NaOH added to the H2SO4 solution. Assume that the volume of the solution does not change. (9)
[16]
QUESTION 8 (Start on a new page.)
8.1 A nickel (Ni) rod is placed in a beaker containing a silver nitrate solution, AgNO3(aq) and a reaction takes place. 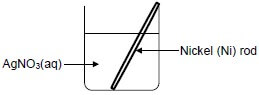
Write down the:
8.1.1 NAME or FORMULA of the electrolyte (1)
8.1.2 Oxidation half-reaction that takes place (2)
8.1.3 Balanced equation for the net (overall) redox reaction that takes place (3)
8.2 A galvanic cell is now set up using a nickel half-cell and a silver half-cell. 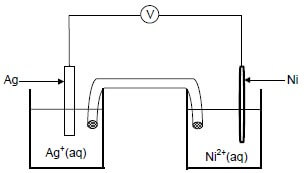
8.2.1 Which electrode (Ni or Ag) must be connected to the negative terminal of the voltmeter? Give a reason for the answer. (2)
8.2.2 Write down the cell notation for the galvanic cell above. (3)
8.2.3 Calculate the initial reading on the voltmeter if the cell functions under standard conditions. (4)
8.2.4 How will the voltmeter reading in QUESTION 8.2.3 be affected if the concentration of the silver ions is increased? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
[16]
QUESTION 9 (Start on a new page.)
In the electrochemical cell below, carbon electrodes are used during the electrolysis of a concentrated sodium chloride solution. 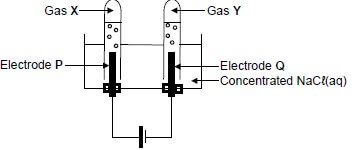
The balanced equation for the net (overall) cell reaction is:
2H2O(ℓ) + 2Cℓ─(aq) → Cℓ2(g) + H2(g) + 2OH─(aq)
9.1 Is the reaction EXOTHERMIC or ENDOTHERMIC? (1)
9.2 Is electrode P the ANODE or the CATHODE? Give a reason for the answer. (2)
9.3 Write down the:
9.3.1 NAME or FORMULA of gas X (1)
9.3.2 NAME or FORMULA of gas Y (1)
9.3.3 Reduction half-reaction (2)
9.4 Is the solution in the cell ACIDIC or ALKALINE (BASIC) after completion of the reaction? Give a reason for the answer. (2)
[9]
QUESTION 10 (Start on a new page.)
10.1 The flow diagram below shows the processes involved in the industrial preparation of fertiliser Q. 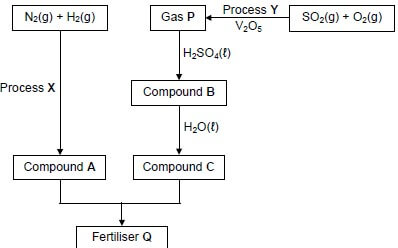
Write down the:
10.1.1 Name of process X (1)
10.1.2 Name of process Y (1)
10.1.3 NAME or FORMULA of gas P (1)
10.1.4 Balanced equation for the formation of compound B (3)
10.1.5 Balanced equation for the formation of fertiliser Q (4)
10.2 The diagram below shows a bag of NPK fertiliser of which the NPK ratio is unknown. It is found that the mass of nitrogen in the bag is 4,11 kg and the mass of phosphorus is 0,51 kg. 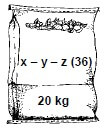
Calculate the NPK ratio of the fertiliser. (4)
[14]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,013 x 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
Charge on electron | e | -1,6 x 10-19 C |
Avogadro's constant | NA | 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE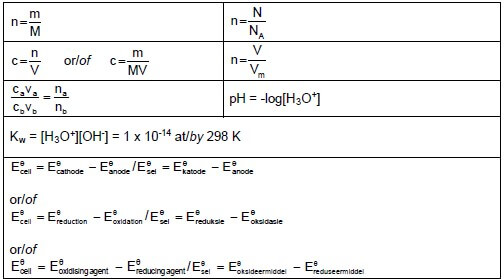
TABLE 3: THE PERIODIC TABLE OF ELEMENTS 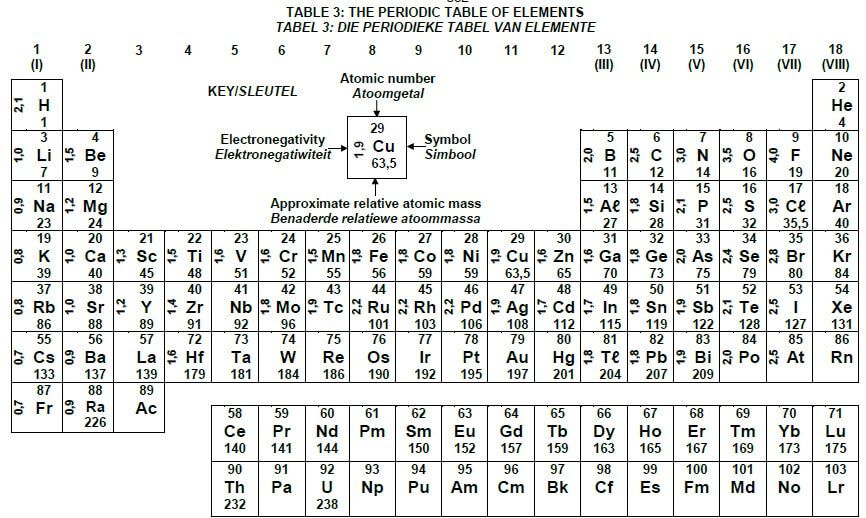
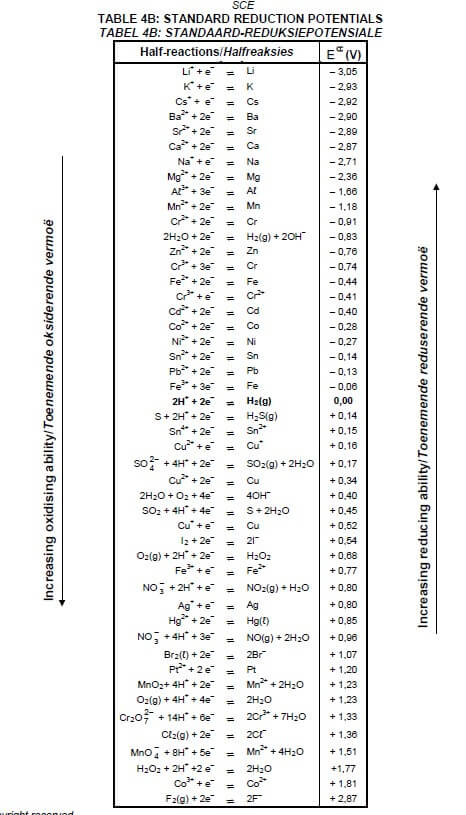
TABLE 4A: STANDARD REDUCTION POTENTIALS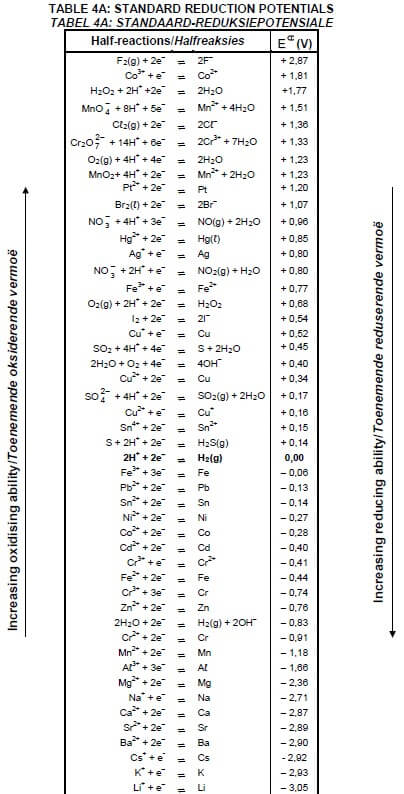
TABLE 4B: STANDARD REDUCTION POTENTIALS