PHYSICAL SCIENCES SCHOOL BASED ASSESSMENT EXEMPLARS - CAPS GRADE 12 TEACHER'S GUIDE
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES
SCHOOL BASED ASSESSMENT EXEMPLARS - CAPS
GRADE 12
TEACHER'S GUIDE
| TABLE OF CONTENT | |||
| CONTENT | PAGE | ||
| 1 | Introduction | 3 | |
| 2 | Objectives | 3 | |
| 3 | Assessment Tasks for Grade 12 Practical Work | 5 | |
| 4 | Quality Assurance Process | 5 | |
| 5 | Exemplars of Practical Work as Formal Assessment Tasks | 6 | |
| 5.1 | Term 1: Preparation of esters and smell identification | 6 | |
| 5.2 | Term 2: Conservation of linear momentum | 10 | |
| 5.3 | Term 3: Electricity and magnetism | ||
| Part 1: Determine the internal resistance of a battery. | 15 | ||
Part 2: Set up a series-parallel network with known resistor. | 17 | ||
| 6 | Memorandum | 18 | |
| 6.1 | Esters and smell identification | 18 | |
| 6.2 | Conservation of linear momentum | 20 | |
| 6.3 | Electricity and magnesium | ||
| Part 1: Internal resistance of a battery | 22 | ||
| Part 2: Equivalent resistance of a series-parallel network | 24 | ||
1. Introduction
Assessment is a continuous planned process of identifying, gathering and interpreting information about the performance of learners, using various forms of assessment. It involves four steps: generating and collecting evidence of achievement; evaluating this evidence; recording the findings and using this information to understand and assist in the learner’s development to improve the process of learning and teaching. Assessment should be both informal (assessment for learning) and formal (assessment of learning). In both cases regular feedback should be provided to learners to enhance the learning experience.
School-based assessment (SBA) is a purposeful collection of learners’ work that tells the story of learner’s efforts, progress or achievement in given area(s). The quality of SBA tasks is integral to learners’ preparation for the final examinations. The SBA component is compulsory for all learners. Learners who cannot comply with the requirements specified according to the policy may not be eligible to enter for the subject in the final examination.
Educators are expected to ensure that assessment tasks are relevant and suitable for the learners. Teachers should adapt the tasks to suit the learners’ level of understanding. Tasks should be context bound. However, they should also take cognisance of the requirements as set out in the Curriculum and Assessment Policy Statement (CAPS)
The CAPS document contains tasks that meet the demands of the Grade 12 Physical Sciences curriculum. It is expected that these tasks will serve as a valuable resource to:
- Physical Sciences teachers, in providing examples of the types and standards of school-based assessment tasks that would be appropriate for their learners
- Grade 12 Physical Science learners, in providing material that will assist them in preparation for the National Senior Certificate examinations
2. The aims and objectives of school-based assessment
- School-based assessment provides a more balanced and trustworthy assessment system, increasing the range and diversity of assessment tasks.
- The exemplar tasks are aimed at reflecting the depth of the curriculum content appropriate for Grade 12.
- They reflect the desired cognitive demands as per Bloom’s revised taxonomy: remembering, understanding, applying, analysing, evaluating and creating.
- School-based assessment improves the validity of assessment by including aspects that cannot be assessed in formal examination settings.
- It improves the reliability of assessment because judgements will be based on many observations of the learner over an extended period of time.
- It empowers teachers to become part of the assessment process and enhances collaboration and sharing of expertise within and across schools.
- It has a professional development function, building up teachers’ skills in assessment practices, which can then be transferred to other areas of the curriculum.
- The tasks focus on the content of the National Curriculum Statement (NCS), as detailed in the Curriculum and Assessment Policy Statement (CAPS) for Physical Sciences effective from 2014 for grade 12.
The distinctive characteristics of SBA (and its strengths as one relatively small component of a coherent assessment system) have implications for its design and implementation, in particular the nature of the assessment tasks and the role of the teacher standardisation procedures. These implications are summarised as follows:
- The assessment process should be linked to and be a logical outcome of the normal teaching programme, as teaching, learning and assessment should be integration of the whole educational experience
- The formative/summative distinction exists in SBA, but is much less rigid and fixed than in a testing culture, i.e. learners should receive constructive feedback and have opportunities to ask questions about specific aspects of their progress after each planned SBA assessment activity.
- The SBA process, to be effective, has to be highly contextualised, dialogic and sensitive to learners’ needs, i.e. the SBA component is not and cannot be treated as identical to an external exam in which texts, tasks and task conditions are totally standardised and all contextual variables controlled. To attempt to do so would be to negate the very rationale for SBA, hence schools and teachers must be granted a certain degree of trust and autonomy in the design, implementation and specific timing of the assessment tasks.
Teachers should ensure that learners understand the assessment criteria. Teachers should also have used these criteria for informal assessment and teaching purposes before they conduct any formal assessments so that they are familiar with the criteria and the assessment process.
Assessment Tasks as outlined by the NCS and CAPS (Physical Sciences)
The final Grade 12 mark is calculated from the National Senior Certificate (NSC) examination that learners will write (out of 300 marks) plus school-based assessment (out of 100 marks).
Physical Sciences investigate physical and chemical phenomena. This is done through scientific enquiry and the application of scientific models, theories and laws in order to explain and predict events in the physical environment.
Practical work in the Physical Sciences must be integrated with theory to strengthen the concepts being taught. These may take the form of simple practical demonstrations or even an experiment or practical investigation.
There are several practical activities outlined in Section 3 (Physical Sciences Content) of the CAPS document. Some of these practical activities will be done as part of formal assessment and others can be done as part of informal assessment. As from 2014 THREE prescribed experiments will be done per year as formal assessment tasks:
- One Chemistry Practical during Term 1
- A Physics or a Chemistry Practical during Term 2
- A Physics Practical during Term 3
Thus THREE practical activities for formal assessment in Grade 12.
(Refer to 3 Assessment Tasks p. 16 as outlined by CAPS.)
3. ASSESSMENT TASKS FOR GRADE 12 PRACTICAL WORK
The table below lists the prescribed formal assessment activities for practical work and the weighting for the annual SBA.
TERM | PRESCRIBED PRACTICAL ACTIVITIES FOR FORMAL ASSESSMENT | WEIGHTING |
1 | EXPERIMENT (CHEMISTRY) | 15% of annual SBA |
2 | EXPERIMENT (CHEMISTRY) EXPERIMENT (PHYSICS) | 20% of annual SBA |
3 | EXPERIMENT (PHYSICS) | 15% of annual SBA |
NOTE: REFER TO THE PROGRAMME OF ASSESSMENT FOR GRADE 12 IN THE CAPS DOCUMENT [Page 148]
4. QUALITY ASSURANCE PROCESS
A team of experts comprising teachers and subject advisors from the provinces was appointed by DBE to develop and compile assessment tasks. This panel of experts spent a period of four days at the DBE developing tasks based on guidelines and policies. Moderation and quality assurance of the tasks were undertaken by national and provincial examiners and moderators. The assessment tasks were further refined by the national internal moderators to ensure that they are in line with the CAPS document.
The purpose of this document is to provide both educators and learners with a set of standardised school-based assessment (SBA) tasks. It contains useful information and guidelines in the form of exemplars.
5. EXEMPLARS OF PRACTICAL WORK AS FORMAL ASSESSMENT TASKS
TERM 1: PRACTICAL WORK
KNOWLEDGE AREA: MATTER AND MATERIALS
5.1 PREPARATION OF ESTERS AND SMELL IDENTIFICATION
Introduction
Esters have a very fruity smell. Naturally occurring esters are found in fruits. Esters can be synthesised by the reaction of a carboxylic acid and an alcohol. This reaction is known as esterification. This reaction can be catalysed by concentrated sulphuric acid.
Aim
- Produce different esters by using a range of carboxylic acids and alcohols.
- Identify the esters formed by their smell.
Apparatus
- Safety goggles
- Test tubes
- Dropping pipettes
- 250 ml beaker
- Test tube rack
- Bunsen burner
- Heat resistant mat/tile
- Tripod
- Wire gauze
- Retort stand
- Chemicals: methanol, ethanol, propanol, ethanoic acid, salicylic acid, sulphuric acid and 0,5 mol.dm-3 sodium carbonate
Method
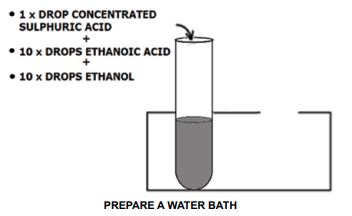
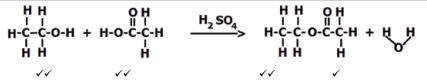
- Place 1 drop of concentrated sulphuric acid in a test tube.
- Add 10 drops of ethanoic acid in the same test tube.
- Add 10 drops of ethanol to the mixture.
PREPARE A WATER BATH
- Pour about 100 cm3 of water into the 250 cm3 beaker.
- Carefully lower the tube into the beaker so that it stands upright.
- Heat the beaker gently on a tripod and gauze until the water begins to boil, and then stop the heating.
- Stand for 1 minute in the hot water. If the mixture in the tube boils, use the tongs to lift it out of the water until the boiling stops, and then return it to the hot water.
- After 1 minute, carefully remove the test tube and allow it to cool.
- When cool, pour the mixture into a test tube half-full of 0,5 mol.dm-3 sodium carbonate solution. There will be some effervescence. Mix well. A layer of ester will separate and float on top of the aqueous layer.
- Smell the product by gently wafting the odour towards your nose with your hand.
- Repeat steps 1 to 10 but use METHANOL and PROPANOL as the alcohol.
- Repeat steps 1 to 10 but use SALICYLIC ACID and METHANOL.
- Results AND INTERPRETATION of results
Complete the tables below.
Choose ONE of the following to identify the ester formed by smell:
- Paint
- Pear
- Pineapple
- Strawberry
- Ice cream
- Nail polish remover
- Wintergreen
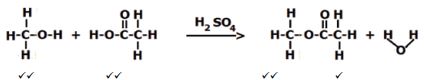
EXPERIMENT 1: ETHANOL + ETHANOIC ACID (16)
SMELL | |
WORD EQUATION | |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION |
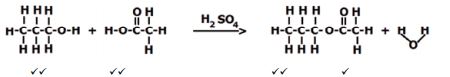
EXPERIMENT 2: METHANOL + ETHANOIC ACID (16)
SMELL | |
WORD EQUATION | |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION |
EXPERIMENT 3: PROPANOL + ETHANOIC ACID (16)
SMELL | |
WORD EQUATION | |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION |
EXPERIMENT 4: METHANOL + SALICYLIC ACID (2)
SMELL |
DISCUSSION OF RESULTS
- Which property of sulphuric acid makes it suitable to use as a catalyst for the preparation of esters? (2)
- Why do we heat the test tube in a water bath and not directly over a flame? (2)
- With reference to the characteristic smells of esters, name TWO examples where esters are used in different industries. (4)
- Why do esters with higher molecular weight not have strong fragrances? (2)
TOTAL: 60
TERM 2: PRACTICAL WORK
KNOWLEDGE AREA: MECHANICS
4.2 CONSERVATION OF LINEAR MOMENTUM
INTRODUCTION
Momentum is mass in motion. The amount of momentum of an object is determined by two variables, namely mass and velocity.
Linear momentum (momentum in a straight line) can be defined as the product of mass and velocity.
The verification of the conservation of momentum can be determined experimentally during an explosion and a collision.
AIM
To verify the conservation of linear momentum during an explosion.
APPARATUS
- Trolley track
- Trolleys
- Meter ruler
- Buffers (wooden plank or brick)
METHOD
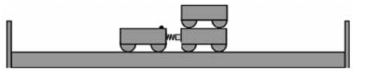
- Place two trolleys, one of which contains a compressed spring, against each other on a smooth, horizontal floor.
- Place another trolley on top of one of the other trolleys in Step 1. These two trolleys now represent a mass of 2 m, while the single trolley represents a mass of m.
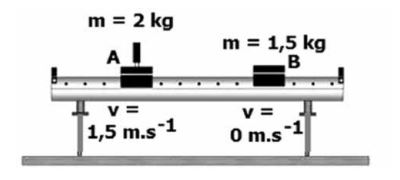
- Place two sturdy wooden planks on either side of the setup (not further than 1–1,5 m from the setup) as shown in the diagram below.
- Release the spring of one trolley so that the two trolley systems move apart. Listen to the collisions against the wooden planks. The trolley systems hit the wooden planks at different times, because one trolley system moves more slowly than the other one (different velocities).
- By means of trial and error, find a position from which the trolley systems move so that both trolleys will hit the wooden planks on both sides at the same time. Only a single collision should be heard.
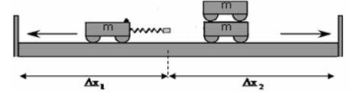
- Measure the distances ∆x1 and ∆x2 that each trolley moved from the starting point to the wooden plank. These distances represent the velocities of the two trolley systems respectively.
- Repeat the above procedure to obtain two more sets of values.
RESULTS
Complete the following table.
Trolley system 1 | Trolley system 2 | Total momentum after explosion (‘unit’) | ||||
Mass (Trolley unit) | [Velocity v1] Distance ∆x1 (cm) | Momentum (‘unit’) | Mass (trolley unit) | [Velocity v2] Distance ∆x2 (cm) | Momentum (‘unit’) | |
(10)
INTERPRETATION AND DISCUSSION OF RESULTS
- Formulate an investigative question for this practical activity. (3)
- State the law of the conservation of momentum. (2)
- Explain why it is acceptable to consider the distances travelled by the trolleys as a measurement of their velocities. (2)
- Give a reason why this experiment must be performed more than once. (2)
CONCLUSION - Draw a conclusion from the results you obtained. (3)
EVALUATION - What recommendations can you make to improve the results of your experiment? (4)
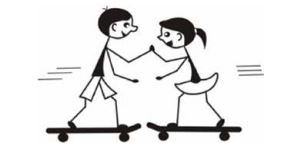
APPLICATION - A boy with a mass of 50 kg and a girl with a mass of 40 kg are standing on skateboards. They press their hands together and push each other apart as shown in the sketch. The girl moves to the right at 1 m.s-1.
7.1 What is the total momentum of the boy and girl before they move apart? (2)
7.2 Determine the velocity of the boy directly after they moved apart. (5)
ALTERNATIVE METHOD – LINEAR AIR TRACK
METHOD
A collision instead of an explosion can be used to investigate the conservation of momentum.
The diagram below illustrates the collision of trolleys on an air track.
Trolley A with a mass of 2 kg and velocity of 1,5 m.s-1 to the right collides with a stationary trolley B with a mass of 1,5 kg.
After the collision trolley A moves at 0,75 m.s-1 to the left and trolley B moves at 3 m.s-1 to the right.
INTERPRETATION OF RESULTS
8.1 In the verification of the conservation of momentum, why is it better to make use an air track rather than a trolley system? (2)
8.2 Prove with a calculation that the momentum was conserved during this collision. (5)
TOTAL: 40
TERM 3: PRACTICAL WORK
KNOWLEDGE AREA: ELECTRICITY AND MAGNETISM
4.3 DETERMINE THE INTERNAL RESISTANCE AND THE EQUIVALENT RESISTANCE OF A SERIES PARALLEL NETWORK
INTRODUCTION
The term ‘lost volts’ refers to the difference between the emf and the terminal voltage. The voltage is not ‘lost’. It is the voltage across the internal resistance of the battery, but ‘lost’ for use in the external circuit.
The internal resistance of the battery can be treated just like another resistor in series in the circuit. The sum of the voltages across the external circuit plus the voltage across the internal resistance is equal to the emf:
ε = Vload + Vinternal resistance or ε = IRexternal + Ir
REARRANGE TO GET: V = -rI + ε
in the form y = mx + c where m = -r
PART 1
Determine the internal resistance of a battery.
AIM
To determine the internal resistance of a battery
APPARATUS
- Voltmeter (or multimeter)
- Ammeter (or multimeter)
- Any size carbon-zinc battery (Choose voltage in relation to the values of the resistors) • Battery holder
- Rheostat
- Connecting wires
- Switch
METHOD
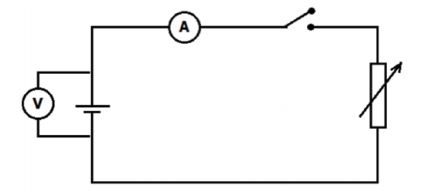
Set up the apparatus as shown in the diagram below and determine the ammeter and voltmeter readings for FIVE different rheostat settings.
PRECAUTION: Do not keep the switch on too long. It will heat the battery and cause it to run down.
RESULTS
- Tabulate the terminal potential difference (volts)- and electric current (amperes) readings obtained from the experiment. (10)
INTERPRETATION AND DISCUSSION OF RESULTS - Identify the:
2.1 Independent variable
2.2 Dependent variable
2.3 Controlled variable (3 x 2) (6) - Why do we include a rheostat in the circuit? (2)
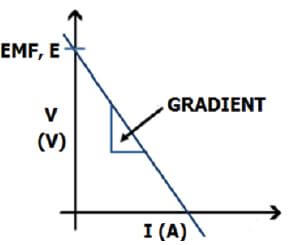
- Draw a graph of the voltmeter readings versus ammeter readings. (8)
- Is the gradient of the graph positive or negative? Explain. (3)
- Use the graph to determine the internal resistance of the battery. (4)
- Which point on your graph represents the emf of the battery? Explain. (4)
CONCLUSION - Draw a conclusion from the results obtained. (2)
PART 2
Set up a series-parallel network with known resistors. Determine the equivalent resistance using an ammeter and a voltmeter and compare with the theoretical value.
AIM
To determine the equivalent resistance of a series-parallel network and compare it with the calculated theoretical value Apparatus
- Three fixed resistors with known values (not too high values)
- Voltmeter (or multimeter)
- Ammeter (or multimeter)
- Battery (choose voltage in relation to the values of the resistors)
- Battery holder
- Connecting wires
- Switch
METHOD
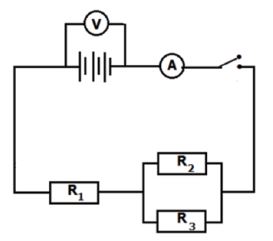
Set up the circuit as shown in the diagram below.
Record the voltmeter and ammeter readings obtained.
INTERPRETATION AND DISCUSSION OF RESULTS
- From the readings obtained in your experiment, determine the equivalent resistance of the circuit. (4)
- By using the values of R1, R2, R3, calculate the theoretical value of the equivalent resistance. (5)
CONCLUSION - Compare the values obtained in 1 and 2 above and draw a suitable conclusion. (2)
TOTAL: 50
6. MEMORANDUM
6.1 PREPARATION OF ESTERS AND SMELL IDENTIFICATION
Results AND INTERPRETATION of results
EXPERIMENT 1: ETHANOL + ETHANOIC ACID (16)
SMELL | nail polish or (pear)✔✔ |
WORD EQUATION | ethanol✔+ ethanoic acid✔? ethyl ethanoate✔+ water✔ |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION | C2H5OH+ CH3COOH ? CH3COOC2H5 + H2O ✔ reactants |
EXPERIMENT 2: METHANOL + ETHANOIC ACID (16)
SMELL | paint✔✔ |
WORD EQUATION | methanol ✔+ ethanoic acid✔ ? methyl ethanoate✔ + water✔ |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION | CH3OH + CH3COOH ? CH3COOCH3 + H2O ✔ reactants |
EXPERIMENT 3: PROPANOL + ETHANOIC ACID (16)
SMELL | pear✔✔ |
WORD EQUATION | propanol✔ + ethanoic acid✔ ? propyl ethanoate✔ + water✔ |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION | C3H7OH + CH3COOH ? CH3COOC3H7 + H2O ✔ reactants |
EXPERIMENT 4: METHANOL + SALICYLIC ACID (2)
SMELL | wintergreen✔✔ |
DISCUSSION OF RESULTS
- Dehydrating characteristic ✔✔ (2)
- Many organic compounds are flammable and should not be left near an open flame. ✔✔ (2)
-
- Food industry: flavouring of food/sweet/also margarine and preservatives
- Cosmetics industry: perfumes/lotions
- Alcohol industry: smell/rum
- Medicine industry: taste of children’s medicine (max 2 x 2) (4)
- Stronger IMF, higher boiling point, lower vapour pressure ✔✔ (2)
TOTAL: 60
6.2 CONSERVATION OF LINEAR MOMENTUM
RESULTS (10)
Trolley system 1 | Trolley system 2 | Total momentum after explosion (‘units’) | ||||
Mass (trolley units) | [Velocity v1] Distance (cm) | Momentum (‘units’) | Mass (trolley units) | [Velocity v2] Distance
| Momentum (‘units’) | |
1 | 125 | 125 | 2 | 62 | - 124 | +1 |
1 | 123 | 123 | 2 | 61 | - 121 | +1 |
2 | 61 | 122 | 2 | 61 | - 122 | 0 |
✔ | ✔ | ✔✔ | ✔ | ✔ | ✔✔ | ✔✔ |
INTERPRETATION AND DISCUSSION OF RESULTS
- Is total momentum before explosion equal to total momentum after explosion?✔✔ (2)
- The total linear momentum in a closed system remains constant in magnitude and direction. ✔✔ (2)
- The distance travelled by each trolley can be calculated with the formula ∆x = v∆t.✔
The times for each trolley to travel their respective distances are the same✔ (they hit the buffers at the same time); therefore the distances can be a measurement of the velocities✔✔✔. (3) - To ensure validity of the results.✔✔ (2)
CONCLUSION - The total linear momentum before the explosion equal/does not equal the total linear momentum after the explosion. (Depending on the results of the learner) ✔✔✔ (3)
EVALUATION -
- Make sure that the masses of the trolleys are identical.
- Make sure that the trolleys are at rest before you start your timing.
- Try to reduce friction (by polishing the track or oiling the wheels of the trolley)
- Do not round off the measured distances (✔✔ + ✔✔ANY TWO) (4)
APPLICATION
-
7.1 0 kg.m.s-1 (2)
7.2- Take right as (+)
p(before explosion) = p(after explosion) ✔
∴ m1vi1 + m2vi2 = m1vf1 + m2vf2
∴ 0 = (50) vi1✔ + (40)(1) ✔
∴ vi1 = –0,8 m∙s–1✔
∴ vi1 = 0,8 m.s-1 in opposite direction from the girl✔ (5)
- Take right as (+)
ALTERNATIVE METHOD - AIR TRACK
1.1 The air track has less friction ✔✔ (2)
1.2 ∑pinitial = mAvAi + mBvBi✔
= (2)(1,5) + 0✔
= 3 kg.m.s-1
∑pfinal = mAvAf + mBvBf
= (2)(-0,75) + (1,5)(3) ✔
= 3 kg.m.s-1✔
∴Momentum before the collision is equal to the momentum after the collision. ✔ (5)
TOTAL: 40
6.3 ELECTRICITY AND MAGNETISM
PART 1: DETERMINE THE INTERNAL RESISTANCE OF A BATTERY.
RESULTS
1. Table for results obtained during experiment
TERMINAL POTENTIAL DIFFERENCE (VOLTS) | ELECTRIC CURRENT (AMPERES) | |
1 | ||
2 | ||
3 | ||
4 | ||
5 |
- CRITERIA FOR MARKING THE TABLE
- Suitable number of columns and rows as dictated by variables✔
- Suitable heading on the columns✔✔
- Correct units for items/quantities recorded in columns✔✔
- Correct sets of values of quantities under investigation✔✔✔✔✔ (10)
INTERPRETATION AND DISCUSSION OF RESULTS
- Variables
2.1 Independent: Terminal potential difference ✔✔
2.2 Dependent: Electric current ✔✔
2.3 Controlled: Temperature ✔✔ (6) - By changing the resistance the flowing current will be changed and the voltage as well. ✔✔ (2)
- Graph

CRITERIA FOR MARKING THE GRAPH- Heading of the graph✔
- Acceptable scale on the two axes✔
- Correct label of the y-axis✔
- Correct unit for the label on the y-axis✔
- Correct label on the x-axis✔
- Correct unit of the label on the x-axis✔
- Correct plotting of points based on captured data✔
- Best fitting line✔ (8)
- Negative.✔
There is decrease✔ in terminal potential difference when electric current increases.✔ (3) - Gradient = ∆V Formula ✔
∆I
= ................. Substitution ✔✔
= ________ Answer ✔ (4) - The y-intercept on the y-axis ✔
When the current is zero✔ the terminal potential difference equals the emf,✔ because there is no internal resistance.✔ (4)
CONCLUSION - The stronger the current the greater the drop in terminal potential difference,✔ therefore the higher the internal resistance of the battery.✔
OR
The internal resistance can be obtained from the gradient of a potential difference versus current graph. (2)
Part 2: DETERMINE THE EQUIVALENT RESISTANCE OF A SERIES-PARALLEL NETWORK
INTERPRETATION AND DISCUSSION OF RESULTS
- R = V/I Formula ✔
= ………………. Substitution ✔✔
= ___________ Answer ✔ (4) - 1 /R// = 1/R2 + 1/R3 Formula ✔
= .......................... Substitution ✔
R// = ___________
RT = R1 + R// Formula
=……… + ………. Substitution ✔✔
= ________ Answer ✔ (5)
CONCLUSION - There is a difference in the values of question 1 and 2 because of the internal resistance of the battery.✔✔
OR
The theoretical value will always differ from the calculated values. The theoretical value does not take cognisance of internal and external factors. (2)
TOTAL: 50