PHYSICAL SCIENCES SCHOOL BASED ASSESSMENT EXEMPLARS - CAPS GRADE 12 LEARNER'S GUIDE
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES
SCHOOL BASED ASSESSMENT EXEMPLARS - CAPS
GRADE 12
LEARNER'S GUIDE
| TABLE OF CONTENT | |||
| CONTENT | PAGE | ||
| 1 | Introduction | 3 | |
| 2 | Objectives | 3 | |
| 3 | Assessment Tasks for Grade 12 Practical Work | 3 | |
| 4 | Exemplars of Practical Work as Formal Assessment Tasks | 4 | |
| 4.1 | Term 1: Preparation of esters and smell identification | 4 | |
| 4.2 | Term 2: Conservation of linear momentum | 9 | |
| 4.3 | Term 3: Electricity and magnetism | ||
| Part 1: Determine the internal resistance of a battery. | 14 | ||
Part 2: Set up a series-parallel network with known resistor. | 16 | ||
1. INTRODUCTION
Assessment of a learner’s progress in Grade 12 Physical Sciences consists of two components:
- A school-based programme of assessment consisting of six tasks, which makes up 25% (mark out of 100) of the total mark for Physical Sciences. Three of the six tasks comprise prescribed experiments.
- An external examination (out of 300 marks), which makes up the remaining 75%.
2. OBJECTIVES
Physical Sciences investigate physical and chemical phenomena. This is done through scientific enquiry and the application of scientific models, theories and laws in order to explain and predict events in the physical environment.
Practical work in Physical Sciences must be integrated with the theory to strengthen the concepts being taught. These may take the form of simple practical demonstrations or even an experiment or practical investigation.
As from 2014 THREE prescribed experiments will be done per year as practical activities for formal assessment:
- One Chemistry Practical during Term 1
- A Physics or a Chemistry Practical during Term 2
- A Physics Practical during Term 3.
This learner guide will provide support to the learner and teacher in performing these practical activities.
3. ASSESSMENT TASKS FOR GRADE 12 PRACTICAL WORK
The table below lists the prescribed formal assessment activities for practical work and the weighting for the annual SBA.
TERM | PRESCRIBED PRACTICAL ACTIVITIES FOR FORMAL ASSESSMENT | WEIGHTING |
1 | EXPERIMENT (CHEMISTRY) | 15% of annual SBA |
2 | EXPERIMENT (CHEMISTRY) EXPERIMENT (PHYSICS) | 20% of annual SBA |
3 | EXPERIMENT (PHYSICS) | 15% of annual SBA |
4. EXEMPLARS OF PRACTICAL WORK AS FORMAL ASSESSMENT TASKS
TERM 1: PRACTICAL WORK
KNOWLEDGE AREA: MATTER AND MATERIALS
4.1 PREPARATION OF ESTERS AND SMELL IDENTIFICATION
Introduction
Esters have a very fruity smell. Naturally occurring esters are found in fruits. Esters can be synthesised by the reaction of a carboxylic acid and an alcohol. This reaction is known as esterification. This reaction can be catalysed by concentrated sulphuric acid.
Aim
- Produce different esters by using a range of carboxylic acids and alcohols.
- Identify the esters formed by their smell.
Apparatus
- Safety goggles
- Test tubes
- Dropping pipettes
- 250 ml beaker
- Test tube rack
- Bunsen burner
- Heat resistant mat/tile
- Tripod
- Wire gauze
- Retort stand
- Chemicals: methanol, ethanol, propanol, ethanoic acid, salicylic acid, sulphuric acid and 0,5 mol.dm-3 sodium carbonate
Method
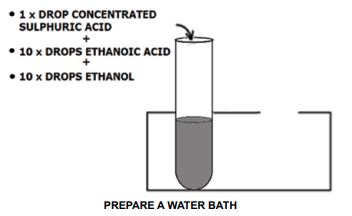
- Place 1 drop of concentrated sulphuric acid in a test tube.
- Add 10 drops of ethanoic acid in the same test tube.
- Add 10 drops of ethanol to the mixture.
PREPARE A WATER BATH
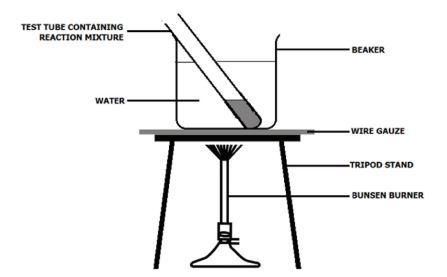
- Pour about 100 cm3 of water into the 250 cm3 beaker.
- Carefully lower the tube into the beaker so that it stands upright.
- Heat the beaker gently on a tripod and gauze until the water begins to boil, and then stop the heating.
- Stand for 1 minute in the hot water. If the mixture in the tube boils, use the tongs to lift it out of the water until the boiling stops, and then return it to the hot water.
- After 1 minute, carefully remove the test tube and allow it to cool.
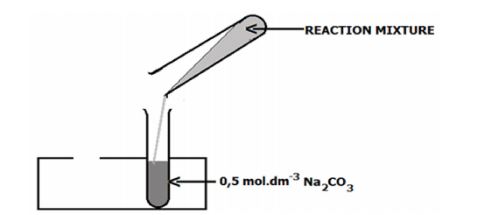
- When cool, pour the mixture into a test tube half-full of 0,5 mol.dm-3 sodium carbonate solution. There will be some effervescence. Mix well. A layer of ester will separate and float on top of the aqueous layer.
- Smell the product by gently wafting the odour towards your nose with your hand.
- Repeat steps 1 to 10 but use METHANOL and PROPANOL as the alcohol.
- Repeat steps 1 to 10 but use SALICYLIC ACID and METHANOL.
- Results AND INTERPRETATION of results
Complete the tables below.
Choose ONE of the following to identify the ester formed by smell:
- Paint
- Pear
- Pineapple
- Strawberry
- Ice cream
- Nail polish remover
- Wintergreen
EXPERIMENT 1: ETHANOL + ETHANOIC ACID (16)
SMELL | |
WORD EQUATION | |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION |
EXPERIMENT 2: METHANOL + ETHANOIC ACID (16)
SMELL | |
WORD EQUATION | |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION |
EXPERIMENT 3: PROPANOL + ETHANOIC ACID (16)
SMELL | |
WORD EQUATION | |
STRUCTURAL FORMULA | |
BALANCED CHEMICAL EQUATION |
EXPERIMENT 4: METHANOL + SALICYLIC ACID (2)
SMELL |
DISCUSSION OF RESULTS
- Which property of sulphuric acid makes it suitable to use as a catalyst for the preparation of esters? (2)
- Why do we heat the test tube in a water bath and not directly over a flame? (2)
- With reference to the characteristic smells of esters, name TWO examples where esters are used in different industries. (4)
- Why do esters with higher molecular weight not have strong fragrances? (2)
TOTAL: 60
TERM 2: PRACTICAL WORK
KNOWLEDGE AREA: MECHANICS
4.2 CONSERVATION OF LINEAR MOMENTUM
INTRODUCTION
Momentum is mass in motion. The amount of momentum of an object is determined by two variables, namely mass and velocity.
Linear momentum (momentum in a straight line) can be defined as the product of mass and velocity.
The verification of the conservation of momentum can be determined experimentally during an explosion and a collision.
AIM
To verify the conservation of linear momentum during an explosion.
APPARATUS
- Trolley track
- Trolleys
- Meter ruler
- Buffers (wooden plank or brick)
METHOD
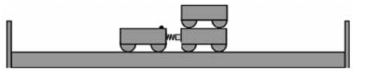
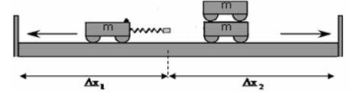
- Place two trolleys, one of which contains a compressed spring, against each other on a smooth, horizontal floor.
- Place another trolley on top of one of the other trolleys in Step 1. These two trolleys now represent a mass of 2 m, while the single trolley represents a mass of m.
- Place two sturdy wooden planks on either side of the setup (not further than 1–1,5 m from the setup) as shown in the diagram below.
- Release the spring of one trolley so that the two trolley systems move apart. Listen to the collisions against the wooden planks. The trolley systems hit the wooden planks at different times, because one trolley system moves more slowly than the other one (different velocities).
- By means of trial and error, find a position from which the trolley systems move so that both trolleys will hit the wooden planks on both sides at the same time. Only a single collision should be heard.
- Measure the distances ∆x1 and ∆x2 that each trolley moved from the starting point to the wooden plank. These distances represent the velocities of the two trolley systems respectively.
- Repeat the above procedure to obtain two more sets of values.
RESULTS
Complete the following table.
Trolley system 1 | Trolley system 2 | Total momentum after explosion (‘unit’) | ||||
Mass (Trolley unit) | [Velocity v1] Distance ∆x1 (cm) | Momentum (‘unit’) | Mass (trolley unit) | [Velocity v2] Distance ∆x2 (cm) | Momentum (‘unit’) | |
(10)
INTERPRETATION AND DISCUSSION OF RESULTS
- Formulate an investigative question for this practical activity. (3)
- State the law of the conservation of momentum. (2)
- Explain why it is acceptable to consider the distances travelled by the trolleys as a measurement of their velocities. (2)
- Give a reason why this experiment must be performed more than once. (2)
CONCLUSION - Draw a conclusion from the results you obtained. (3)
EVALUATION - What recommendations can you make to improve the results of your experiment? (4)
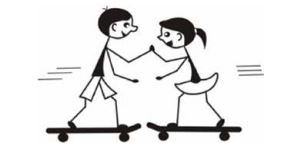
APPLICATION - A boy with a mass of 50 kg and a girl with a mass of 40 kg are standing on skateboards. They press their hands together and push each other apart as shown in the sketch. The girl moves to the right at 1 m.s-1.
7.1 What is the total momentum of the boy and girl before they move apart? (2)
7.2 Determine the velocity of the boy directly after they moved apart. (5)
ALTERNATIVE METHOD – LINEAR AIR TRACK
METHOD
A collision instead of an explosion can be used to investigate the conservation of momentum.
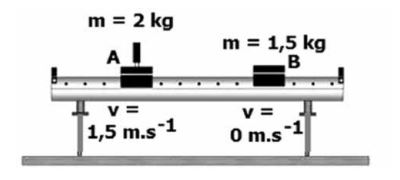
The diagram below illustrates the collision of trolleys on an air track.
Trolley A with a mass of 2 kg and velocity of 1,5 m.s-1 to the right collides with a stationary trolley B with a mass of 1,5 kg.
After the collision trolley A moves at 0,75 m.s-1 to the left and trolley B moves at 3 m.s-1 to the right.
INTERPRETATION OF RESULTS
8.1 In the verification of the conservation of momentum, why is it better to make use an air track rather than a trolley system? (2)
8.2 Prove with a calculation that the momentum was conserved during this collision. (5)
TOTAL: 40
TERM 3: PRACTICAL WORK
KNOWLEDGE AREA: ELECTRICITY AND MAGNETISM
4.3 DETERMINE THE INTERNAL RESISTANCE AND THE EQUIVALENT RESISTANCE OF A SERIES PARALLEL NETWORK
INTRODUCTION
The term ‘lost volts’ refers to the difference between the emf and the terminal voltage. The voltage is not ‘lost’. It is the voltage across the internal resistance of the battery, but ‘lost’ for use in the external circuit.
The internal resistance of the battery can be treated just like another resistor in series in the circuit. The sum of the voltages across the external circuit plus the voltage across the internal resistance is equal to the emf:
ε = Vload + Vinternal resistance or ε = IRexternal + Ir
REARRANGE TO GET: V = -rI + ε
in the form y = mx + c where m = -r
PART 1
Determine the internal resistance of a battery.
AIM
To determine the internal resistance of a battery
APPARATUS
- Voltmeter (or multimeter)
- Ammeter (or multimeter)
- Any size carbon-zinc battery (Choose voltage in relation to the values of the resistors) • Battery holder
- Rheostat
- Connecting wires
- Switch
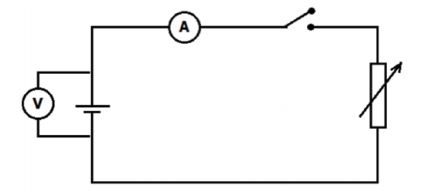
METHOD
Set up the apparatus as shown in the diagram below and determine the ammeter and voltmeter readings for FIVE different rheostat settings.
PRECAUTION: Do not keep the switch on too long. It will heat the battery and cause it to run down.
RESULTS
- Tabulate the terminal potential difference (volts)- and electric current (amperes) readings obtained from the experiment. (10)
INTERPRETATION AND DISCUSSION OF RESULTS - Identify the:
2.1 Independent variable
2.2 Dependent variable
2.3 Controlled variable (3 x 2) (6) - Why do we include a rheostat in the circuit? (2)
- Draw a graph of the voltmeter readings versus ammeter readings. (8)
- Is the gradient of the graph positive or negative? Explain. (3)
- Use the graph to determine the internal resistance of the battery. (4)
- Which point on your graph represents the emf of the battery? Explain. (4)
CONCLUSION - Draw a conclusion from the results obtained. (2)
PART 2
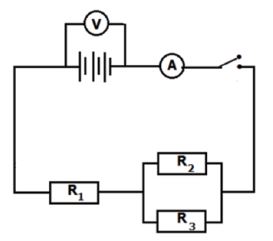
Set up a series-parallel network with known resistors. Determine the equivalent resistance using an ammeter and a voltmeter and compare with the theoretical value.
AIM
To determine the equivalent resistance of a series-parallel network and compare it with the calculated theoretical value Apparatus
- Three fixed resistors with known values (not too high values)
- Voltmeter (or multimeter)
- Ammeter (or multimeter)
- Battery (choose voltage in relation to the values of the resistors)
- Battery holder
- Connecting wires
- Switch
METHOD
Set up the circuit as shown in the diagram below.
Record the voltmeter and ammeter readings obtained.
INTERPRETATION AND DISCUSSION OF RESULTS
- From the readings obtained in your experiment, determine the equivalent resistance of the circuit. (4)
- By using the values of R1, R2, R3, calculate the theoretical value of the equivalent resistance. (5)
CONCLUSION - Compare the values obtained in 1 and 2 above and draw a suitable conclusion. (2)
TOTAL: 50