PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
INSTRUCTIONS AND INFORMATION
- Write your examination number and centre number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your final numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Write down the question number (1.1–1.10), choose the answer and make a cross (X) over the letter (A–D) of your choice in the ANSWER BOOK.
EXAMPLE:
1.11 ![]()
1.1 Which ONE of the following is the product formed in the Haber process?
- Nitrogen
- Ammonia
- Nitric acid
- Sulphuric acid (2)
1.2 A carbonyl group is the functional group of …
- alcohols.
- ketones.
- haloalkanes.
- carboxylic acids. (2)
1.3 Consider the structure of an organic compound below. 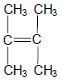
The IUPAC name of this compound is …
- 2,3-dimethylbut-2-ene.
- 2,2-dimethylbut-2-ene.
- 1,1,2-trimethylprop-1-ene.
- 1,1,2,2-tetramethylethene. (2)
1.4 Consider the reaction represented below.
CH3CH2CH2CH2CH3 → CH3CHCH2 + X
Which ONE of the following CORRECTLY gives the type of reaction that takes place and the IUPAC name of product X?
Type of reaction | Product X | |
A | Elimination | Ethane |
B | Elimination | Ethene |
C | Addition | Ethane |
D | Addition | Ethene |
(2)
1.5 Consider the following balanced equation of a chemical reaction:
2NaCℓ + 2H2O → Cℓ2 + H2 + 2NaOH
Which ONE of the following statements about the reaction is correct?
The reaction takes place in a/an …
- galvanic cell and absorbs energy.
- galvanic cell and releases energy.
- electrolytic cell and absorbs energy.
- electrolytic cell and releases energy. (2)
1.6 The following equation represents the reaction taking place in an electrochemical cell:
Ni(s) + Pb2+(aq) → Ni2+(aq) + Pb(s)
The flow of electrons through the external circuit of this cell is from …
- Pb at the anode to Ni at the cathode.
- Pb at the cathode to Ni at the anode.
- Ni at the cathode to Pb at the anode.
- Ni at the anode to Pb at the cathode. (2)
1.7 A solution has a pH = 1. This solution …
- contains no OH─ ions.
- neutralises a hydrochloric acid solution of pH = 1.
- contains a higher concentration of H3O+ ions than OH─ ions.
- contains a higher concentration of OH─ ions than H3O+ ions. (2)
1.8 A potential energy diagram can be used to show the activation energy (EA) and the heat of reaction (ΔH) of a reaction.
Which ONE of the following combinations of values of EA and ΔH CANNOT be obtained for any reaction?
EA (kJ·mol-1) | ∆H (kJ·mol-1) | |
A | 50 | -100 |
B | 50 | +100 |
C | 100 | +50 |
D | 100 | -50 |
(2)
1.9 Initially, 2 mol CO(g) and 2 mol H2(g) are sealed in a container. The reaction reaches equilibrium according to the following balanced equation:
CO(g) + 2H2(g) ⇌ CH3OH(g)
At equilibrium the amount of CH3OH(g) in the mixture will be …
- 1 mol.
- 2 mol.
- less than 1 mol.
- greater than 1 mol. (2)
1.10 The graph below represents the change in concentration of a reactant against time for a chemical reaction. 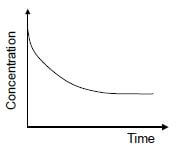
In which ONE of the following graphs does the dotted line show the effect of a catalyst on this reactant? 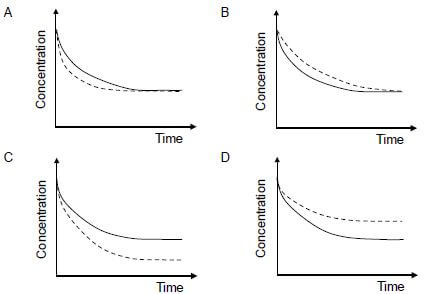 (2)
(2)
[20]
QUESTION 2 (Start on a new page.)
The letters A to F in the table below represent six organic compounds. 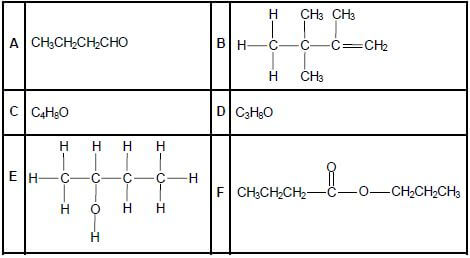
2.1 Write down the letter that represents EACH of the following:
2.1.1 A hydrocarbon (1)
2.1.2 An alcohol (1)
2.1.3 An ester (1)
2.2 Write down the IUPAC name of:
2.2.1 Compound A (1)
2.2.2 Compound B (3)
2.3 Compound C is a functional isomer of compound A. Write down the structural formula of compound C. (2)
2.4 Compound D is used as one of the reactants to prepare compound F. Write down the:
2.4.1 Type of reaction which takes place to prepare compound F (1)
2.4.2 IUPAC name of compound D (2)
2.4.3 Structural formula of the other organic reactant used (2)
2.4.4 IUPAC name of compound F (2)
[16]
QUESTION 3 (Start on a new page.)
Learners investigate factors which influence the boiling points of alcohols.
They use equal volumes of each of the alcohols and heat them separately in a water bath. The temperature at which each boils is measured. The results obtained are shown in the table below.
ALCOHOLS | BOILING POINTS OF ALCOHOLS |
Butan-1-ol | 117,7 |
Pentan-1-ol | 138,5 |
Hexan-1-ol | 157,0 |
3.1 Define the term boiling point. (2)
3.2 What property of alcohols requires them to be heated in a water bath? (1)
3.3 The boiling points of the alcohols are compared with each other.
3.3.1 What structural requirements must the alcohols meet to make it a fair comparison? (2)
3.3.2 Fully explain the trend in the boiling points. (3)
3.4 How will the boiling point of hexan-1-ol be affected if the volume of hexan-1-ol used is doubled? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
3.5 In another investigation the learners compare the boiling points of hexan-1-ol and hexanal.
3.5.1 Write down the independent variable for this comparison. (1)
3.5.2 They find that the boiling point of hexan-1-ol is higher than that of hexanal. Fully explain this observation. (4)
[14]
QUESTION 4 (Start on a new page.)
4.1 Consider the reactions represented in the flow diagram below.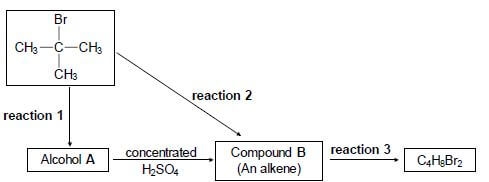
Write down the:
4.1.1 Type of reaction represented by reaction 1 (1)
4.1.2 NAME or FORMULA of the inorganic reactant needed for reaction 1 (1)
4.1.3 Type of alcohol (PRIMARY, SECONDARY or TERTIARY) of which alcohol A is an example (1)
4.1.4 Type of reaction represented by reaction 2 (1)
4.1.5 IUPAC name of compound B (2)
4.1.6 Type of addition reaction represented by reaction 3 (1)
4.1.7 Balanced equation for reaction 3 using structural formulae (4)
4.2 A wide range of synthetic polymers are produced by combining large numbers of similar small organic molecules bonded to each other in a repeating pattern.
Polymer C below is an example of such a polymer. 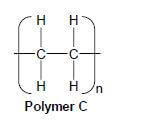
Write down:
4.2.1 ONE word for the underlined phrase (1)
4.2.2 The homologous series to which the 'small organic molecules' used to produce polymer C belong (1)
4.2.3 The type of polymerisation which takes place to produce polymer C (1)
[14]
QUESTION 5 (Start on a new page.)
The reaction of copper(II) carbonate with excess dilute hydrochloric acid is used to investigate the rate of reaction. The balanced equation for the reaction is:
CuCO3(s) + 2HCℓ(aq) → CuCℓ2(aq) + H2O(ℓ) + CO2(g)
The apparatus used is illustrated below. 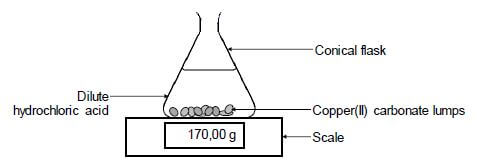
5.1 State TWO ways in which the rate of the reaction above can be increased. (2)
During the investigation, samples of both PURE and IMPURE copper(II) carbonate of EQUAL mass are used. The graphs below are obtained from the results. 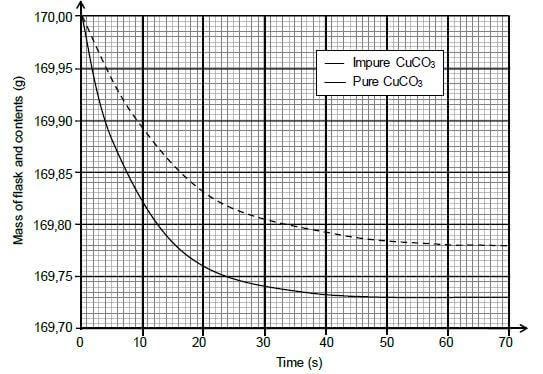
5.2 Write down the reaction time for the reaction of the pure CuCO3 with HCℓ. (1)
5.3 Assume that all the gas formed during the two reactions escape from the flask and that the impurities do not react.
Calculate the:
5.3.1 Average rate of the reaction of the pure sample over the first 20 s (3)
5.3.2 Percentage purity of the impure sample (4)
5.3.3 Maximum volume of CO2(g) produced during the reaction of the pure sample of CuCO3 if the reaction takes place at STANDARD CONDITIONS (3)
5.4 Sketch a graph of the volume of gas produced versus time for the reaction of the pure CuCO3. Indicate the reaction time on the x-axis. (2)
[15]
QUESTION 6 (Start on a new page.)
Hydrogen and iodine are sealed in a 2 dm3 container. The reaction is allowed to reach equilibrium at 700 K according to the following balanced equation:
H2(g) + I2(g) ⇌ 2HI(g)
6.1 Give a reason why changes in pressure will have no effect on the equilibrium position. (1)
6.2 At equilibrium, 0,028 mol H2(g) and 0,017 mol I2(g) are present in the container.
Calculate the initial mass of I2(g), in grams, that was sealed in the container, if Kc for the reaction is 55,3 at 700 K. (9)
The reaction rate versus time graph below represents different changes made to the equilibrium mixture. 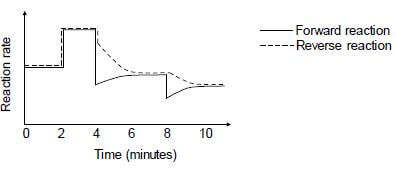
6.3 What do the parallel lines in the first two minutes indicate? (1)
6.4 State TWO possible changes that could be made to the reaction conditions at t = 2 minutes. (2)
6.5 The temperature of the equilibrium mixture was changed at t = 4 minutes.
6.5.1 Is the forward reaction EXOTHERMIC or ENDOTHERMIC? Fully explain the answer. (3)
6.5.2 How will this change influence the Kc value? Choose from INCREASES, DECREASES or REMAINS THE SAME. (1)
6.6 What change was made to the equilibrium mixture at t = 8 minutes? (1)
[18]
QUESTION 7 (Start on a new page.)
The Ka values for two weak acids, oxalic acid and carbonic acid, are as follows:
NAME | FORMULA | Ka |
Oxalic acid | (COOH)2 | 5,6 x 10-2 |
Carbonic acid | H2CO3 | 4,3 x 10-7 |
7.1 Define the term weak acid. (2)
7.2 Which acid, OXALIC ACID or CARBONIC ACID, is stronger? Give a reason for the answer. (2)
7.3 Oxalic acid ionises in water according to the following balanced equation:
(COOH)2(s) + 2H2O(ℓ) ⇌ (COO)2−2 (aq) + 2H3O+(aq)
Write down the FORMULAE of the TWO bases in this equation. (2)
7.4 Learners prepare 2 dm3 of a sodium hydroxide solution of concentration 0,1 mol∙dm-3. Calculate the pH of the solution. (4)
7.5 During a titration of the sodium hydroxide solution in QUESTION 7.4 with dilute oxalic acid, the learners find that 25,1 cm3 of the NaOH(aq) neutralises exactly 14,2 cm3 of the (COOH)2(aq).
The balanced equation for the reaction is as follows:
2NaOH(aq) + (COOH)2(aq) → (COO)2Na2(aq) + 2H2O(ℓ)
7.5.1 Calculate the concentration of the oxalic acid solution. (5) The following indicators are available for the titration:
INDICATOR | pH RANGE |
A | 3,1–4,4 |
B | 6,0–7,6 |
C | 8,3–10,0 |
7.5.2 Which ONE of the indicators above is most suitable for this titration? Give a reason for the answer. (2)
[17]
QUESTION 8 (Start on a new page.)
In the electrochemical cell shown below an aluminium electrode and another metal electrode, Y, are used. 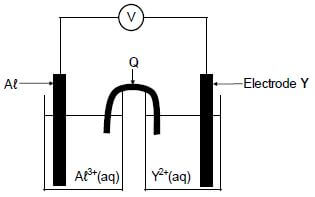
8.1 Write down the:
8.1.1 Name of component Q (1)
8.1.2 Type of electrochemical cell represented above (1)
It is found that the mass of the aluminium electrode increases whilst the cell is functioning.
8.2 How will EACH of the following change while the cell is functioning? Choose from INCREASES, DECREASES or REMAINS THE SAME.
8.2.1 The concentration of Aℓ3+(aq) (1)
8.2.2 The concentration of Y2+(aq) (1)
8.3 Write down the:
8.3.1 Half-reaction that takes place at electrode Y (2)
8.3.2 Cell notation of the cell (3)
8.4 The initial emf of this cell measured under standard conditions is 0,7 V. Identify metal Y by means of a calculation. (5)
[14]
QUESTION 9 (Start on a new page.)
The simplified diagram below shows an electrolytic cell used in the industrial extraction of aluminium (Aℓ) from aluminium oxide at temperatures as high as 1 000 ºC.
Electrode X is a carbon rod. 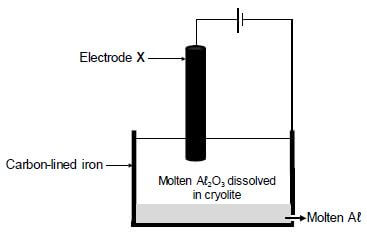
The cell reaction that takes place is as follows:
2Aℓ2O3(ℓ) → 4Aℓ(ℓ) + 3O2(g)
9.1 Write down the name of the ore used as source of aluminium oxide. (1)
9.2 Which half-reaction (OXIDATION or REDUCTION) takes place at electrode X? (1)
9.3 What is the function of the cryolite? (1) 9.4 Write down the reduction half-reaction. (2)
9.5 Write down a balanced equation that shows why the carbon rod, X, must be replaced regularly. (3)
[8]
QUESTION 10 (Start on a new page.)
10.1 The reactions represented below take place during one of the industrial processes used in the fertiliser industry.
PT
- : 4NH3(g) + 5O2(g) ⇌ 4NO(g) + 6H2O(g) ∆H < 0
- NO(g) + O2(g) ⇌ X
- : NO2 + H2O(ℓ) ⇌ HNO3(aq) + _____
Write down:
10.1.1 The name of this industrial process (1)
10.1.2 The function of Pt in reaction I (1)
10.1.3 The NAME of product X (1)
10.1.4 A balanced equation for reaction III (2)
10.1.5 TWO ways in which the yield of the NO(g) obtained in reaction I can be increased without changing the amount of reactants and products (2)
10.2 NPK fertilisers contain NH4NO3, (NH4)3PO4 and KCℓ in varying proportions.
10.2.1 What does NPK mean? (1)
10.2.2 Consider the fertiliser illustrated below. 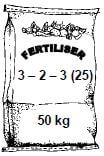
Calculate the mass, in kg, of KCℓ needed to produce this fertiliser. (6)
[14]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12
PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
NAME | SYMBOL | VALUE |
Standard pressure | pθ | 1,013 x 105 Pa |
Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
Standard temperature | Tθ | 273 K |
Charge on electron | e | -1,6 x 10-19 C |
Avogadro's constant | NA | 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE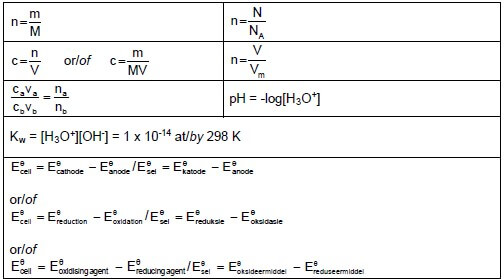
TABLE 3: THE PERIODIC TABLE OF ELEMENTS 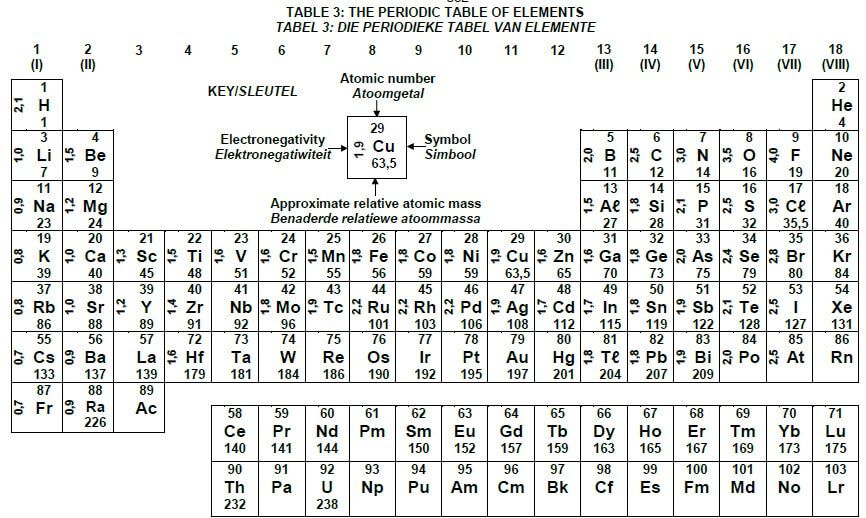
TABLE 4A: STANDARD REDUCTION POTENTIALS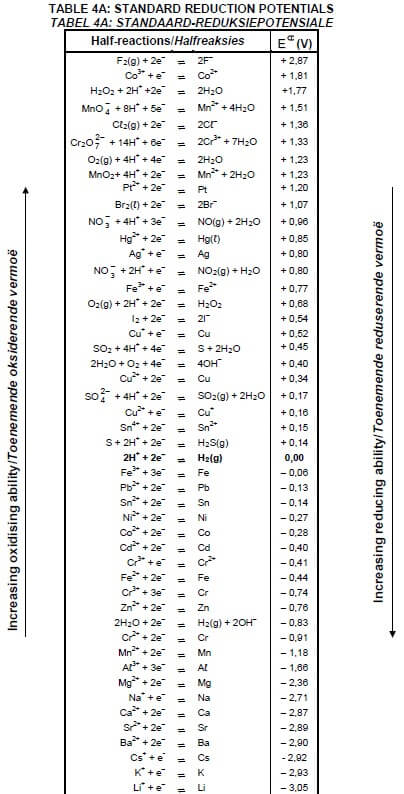
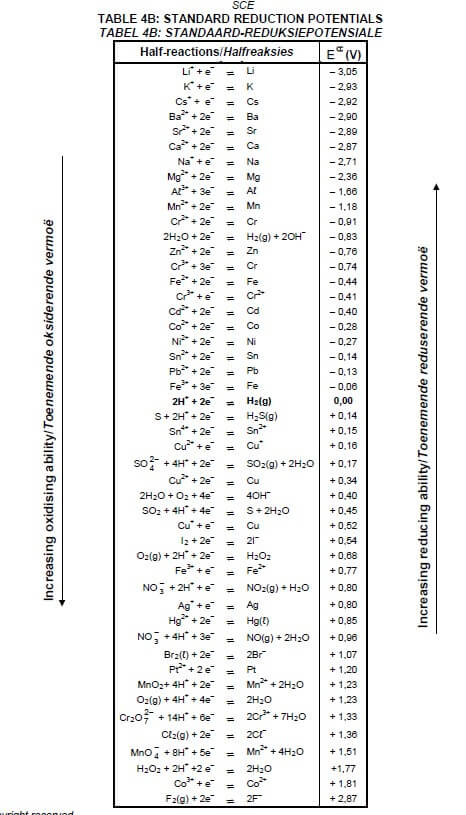
TABLE 4B: STANDARD REDUCTION POTENTIALS