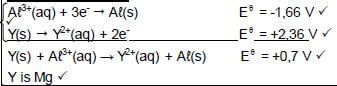PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 MEMORANDUM - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2017
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2017
MEMORANDUM
QUESTION 1
1.1 B ✔✔ (2)
1.2 B ✔✔ (2)
1.3 A ✔✔ (2)
1.4 A ✔✔ (2)
1.5 C ✔✔ (2)
1.6 D ✔✔ (2)
1.7 C ✔✔ (2)
1.8 B ✔✔ (2)
1.9 C ✔✔ (2)
1.10 A ✔✔ (2)
[20]
QUESTION 2
2.1
2.1.1 B ✔ (1)
2.1.2 D OR/OF E ✔ (1)
2.1.3 F ✔ (1)
2.2
2.2.1 Butanal ✔ (1)
2.2.2 2,3,3-trimethyl✔but-1-ene ✔
Accept
2,3,3- trimethyl ✔-1- butene
Marking criteria:
|
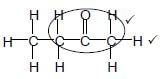 (3)
(3)
Marking criteria:
|
(2)
2.4
2.4.1 Esterification / Condensation ✔ (1)
2.4.2 Propan-1-ol ✔ ✔
If propanol (1 mark) (2)
2.4.3 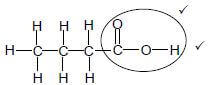
Marking criteria:
|
(2)
2.4.4 Propyl ✔ butanoate ✔(2)
[16]
QUESTION 3
3.1
- The temperature at which the vapour pressure equals atmospheric (external) pressure. ✔✔ (2 or 0) (2)
3.2
- Flammable / Catch fire easily. / Volatile ✔ (1)
3.3
3.3.1
- Use straight chain✔ primary alcohols ✔ (2)
3.3.2. OPTION 1
- Structure
Chain length / more C atoms in chain / molecular size / molecular mass / surface area increases from top to bottom / butan-1-ol to hexan-1-ol.✔ - Intermolecular forces
Intermolecular forces / Van der Waals forces / London forces / dispersion forces increases from top to bottom / butan-1-ol to hexan-1-ol. ✔ - Energy
Energy needed to overcome / break intermolecular forces increases from top to bottom / butan-1-ol to hexan-1-ol. ✔
OPTION 2
- Structure
Chain length / number of C atoms in the chain / molecular size / molecular mass/surface area decreases from bottom to top / hexan-1-ol to butan-1-ol. ✔ - Intermolecular forces
Intermolecular forces / Van der Waals forces/London forces / dispersion forces decreases from bottom to top/hexan-1-ol to butan-1-ol. ✔ - Energy
Energy needed to overcome / break intermolecular forces decreases from bottom to top / hexan-1-ol to butan-1-ol. ✔ (3)
3.4 Remains the same / Bly dieselfde ✔ (1)
3.5
3.5.1 Functional group / Type of homologous series ✔ (1)
3.5.2
- Type of intermolecular forces
Between molecules of aldehyde / hexanal are dipole-dipole forces. ✔ - Between molecules of alcohols / hexan-1ol are (in addition to dipole-dipole forces and London forces) hydrogen bonds. ✔
- Strength of intermolecular forces
Dipole-dipole forces are weaker than hydrogen bonds. ✔
OR
Hydrogen bonds are stronger than dipole-dipole forces. - Energy
More energy needed to overcome / break intermolecular forces in hexan-1-ol. ✔
OR
Less energy needed to overcome / break intermolecular forces in hexanal.✔ (4)
[14]
QUESTION 4
4.1
4.1.1 Substitution / hydrolysis ✔ (1)
4.1.2 H2O/water ✔
OR
Dilute sodium hydroxide /NaOH(aq)
OR
Dilute potassium hydroxide/KOH(aq)(1)
4.1.3 Tertiary ✔ (1)
4.1.4 Elimination / dehydrohalogenation / dehydrobromination ✔ (1) ✔✔
4.1.5 2-methylprop-1-ene / methylpropene / 2-methylpropene (2)
4.1.6 Halogenation / bromination ✔ (1)
4.1.7 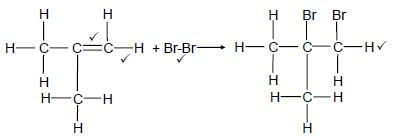 (4)
(4)
|
Notes:
|
4.2
4.2.1 Monomers ✔ (1)
4.2.2 Alkenes ✔ (1)
4.2.3 Addition (polymerisation) ✔ (1)
[14]
QUESTION 5/VRAAG 5
5.1 ANY TWO:
- Increase temperature of HCℓ. ✔
- Add a catalyst ✔
- Increase the concentration of HCℓ. ✔
- Increase the state of division of CuCO3 ✔
- Agitation / Stirring ✔ (2)
5.2 Accepted range : 42 s to 50 s ✔ (1)
5.3
5.3.
- average = - Δ m
Δ t
= - (169,76 - 170,000)✔
(20 - 0) ✔
= 0,012(g.s-1)✔
If answer is negative (minus 1 mark) (3)
5.3.2 Pure sample:
m(CO2)formed = 170,00 – 169,73 ✔
= 0,27 g
Impure sample:
m(CO2)formed = 170,00 - 169,78 ✔
= 0,22 g
%Purity = 0,22 × 100 ✔
0,27
= 81,48% ✔ (4)
5.3.3 POSITIVE MARKING FROM QUESTION 5.3.2.
- n(CO2) formed = m
M
= 0,27
44
= 6,13 × 10-3 mol
n(CO2) formed = V
VM
6,13 × 10-3 = V
22,4
V = 0,137 dm3 (3)
5.4 POSITIVE MARKING FROM QUESTION 5.2. 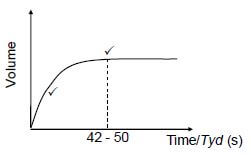
| Marking criteria for sketch graph: | |
Graph drawn from origin with decreasing gradient. | ✔ |
Constant volume after (42 -50) s.or graph stops at (42 -50) s | ✔ |
If no labels on axes: minus 1. |
(2)
[15]
QUESTION 6
6.1 Amount / number of moles / volume of (gas) reactants equals amount/number of moles/volume of (gas) products. ✔
OR
A change in pressure will change the concentration of the reactants and products equally. (1)
6.2
CALCULATIONS USING NUMBER OF MOLES
|
OPTION 1
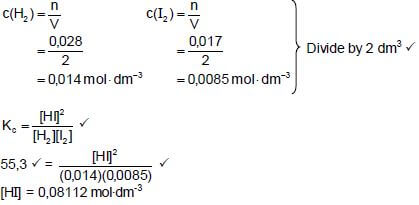
KC = [HI]2
[H2][I2]
∴55,3 = [HI]2
(0,014)(0,0085)
∴[HI] = 0,08112 mol.dm-3
| No Kc expression, correct substitution Max. 8/9 | ||||
| Wrong Kc expression Max.6/9 | ||||
H2 | I2 | HI | ||
Initial mass (g) | (0,09812)(254) ✔ | |||
Initial quantity (mol) | 0,1091 | 0,09812 | 0 | |
Change (mol) | 0,08112 | 0,08112 ✔ | 0,1622 ✔ | using ratio |
Quantity at equilibrium (mol)/ | 0,028 | 0,017 | 0,1622 | |
Equilibrium concentration (mol∙dm-3) | 0,014 | 0,0085 | 0,08112 | × 2 |
| Divide by 2 ✔ | ||||
OR
KC = [HI]2
[H2][I2]
∴55,3 = X2
(0,014)(0,0085)
∴X = 0,08112 mol.dm-3
| No Kc expression, correct substitution Max. 8/9 | ||||
| Wrong Kc expression Max.6/9 | ||||
H2 | I2 | HI | ||
Initial mass (g) |
| |||
Initial quantity (mol) | x + 0,028 | x + 0,017 | 0 | |
Change (mol) | x | x | 2x | using ratio |
Quantity at equilibrium (mol)/ | 0,028 | 0,017 | 2x | |
Equilibrium concentration (mol∙dm-3) | 0,014 | 0,0085 | x | × 2 |
| Divide by 2 ✔ | ||||
Initial quantity I2(mol) (mol) = 0,08112 + 0,017
= 0,09812 mol
m(I2) = nM
= (0,09812)(254) ✔
= 24,92 g ✔
OPTION 2
n(HI at equilibrium) = (0,08112)(2) = 0,1622 mol
n(HI formed) = n(HI at equilibrium) = 0,1622 mol
n(I2 reacted) = ½n(HI formed) = 0,08112 mol
n(I2 initial) = n(I2 reacted) + n(I2 equilibrium)
= 0,08112 + 0,017
= 0,09812 mol
m(I2 initial) = nM
= (0,09812)(254)
= 24,92 (g)✔
CALCULATIONS USING CONCENTRATION
|
OPTION 3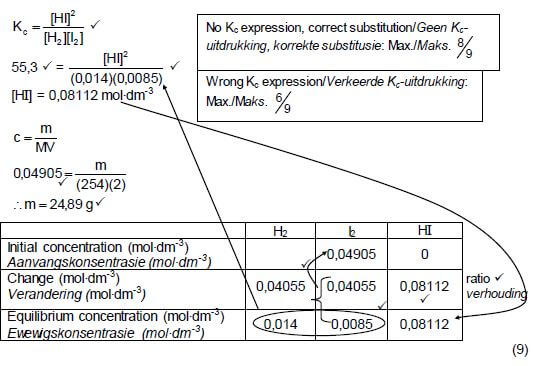
6.3 (Chemical/dynamic) equilibrium
OR
The rate of the forward reaction equals the rate of the reverse reaction. ✔ (1)
6.4
- Addition of a catalyst.
- Increase in pressure. (2)
6.5.1 Endothermic
- The rate of the forward reaction decreases more. / The rate of the reverse reaction decreases less.
- A decrease in temperature favours the exothermic reaction. (3)
6.5.2 Decreases (1)
6.6 Reactants / H2 / I2 removed
[18]
QUESTION 7
7.1 A substance that ionises incompletely/to a small extent.(2)
7.2
- Oxalic acid
- Higher Ka value
OR - Carbonic acid has a lower Ka value .(2)
7.3
- H2O
- (COO)2−2 (2)
7.4  (4)
(4)
7.5
7.5.1 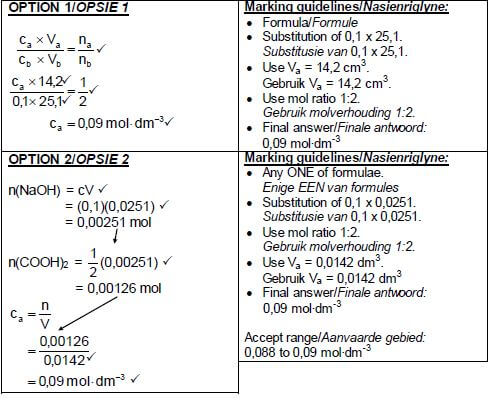 (5)
(5)
7.5.2
- C / phenolphthalein
- Titration of weak acid and strong base.
OR - The endpoint will be at pH > 7 which is in the range of the indicator. (2)
[17]
QUESTION 8
8.1
8.1.1 Salt bridge (1)
8.1.2 Voltaic / Galvanic cell (1)
8.2
8.2.1 Decreases(1)
8.2.2 Increases (1)
8.3
8.3.1 Y(s) → Y2+(aq) + 2e- Ignore phases
OR
Mg(s) → Mg2+(aq) + 2e -
Notes 0) Y2+(aq) + 2e- ⇌ Y(s) (0/2) Y(s) ← Y2+(aq) + 2e- (0/2) |
(2)
8.3.2 Y(s) |Y2+(aq) ||Aℓ3+(aq) | Aℓ(s) OR/OF Mg(s) |Mg2+(aq) ||Aℓ3+(aq) | Aℓ(s
OR
Y(s) | Y2+ (1 mol∙dm-3) || Aℓ3+(1 mol∙dm-3) | Aℓ(s)
Accept
Y | Y2+ || Aℓ3+ | Aℓ (3)
8.4 (5)
OPTION 1 0,7 = -1, 66 - Eθoxidation Eθoxidation = -2,36 (V) Y is Mg | Notes
|
OPTION 2
| |
[14]
QUESTION 9
9.1 Bauxite (1)
9.2 Oxidation (1)
9.3 Reduce melting point .
OR
To lower the temperature / energy needed to melt the Aℓ2O3. (1)
ACCEPT
|
9.4 Aℓ3+(aq) + 3e- → Aℓ(s)
Ignore phases
Notes |
(2)
9.5
- C + O2 → CO2 Bal
OR - 2Aℓ2O3 + 3C → 4Aℓ + 3CO2 Bal
Notes
|
(3)
[8]
QUESTION 10/VRAAG 10
10.1
10.1.1 Ostwald (process) (1)
10.1.2 Catalyst/Speeds up the rate of the reaction (1)
10.1.3 Nitrogen dioxide (1)
10.1.4 3NO2 + H2O ⇌ 2HNO3(aq) + NO Bal. (2)
Notes:
|
10.1.5
- Decrease pressure / Increase volume
- Decrease temperature \ (2)
10.2
10.2.1 (Ratio of the) nitrogen, phosphorous and potassium in the fertiliser. (1)
10.2.2 (6)
Marking criteria:
| ||
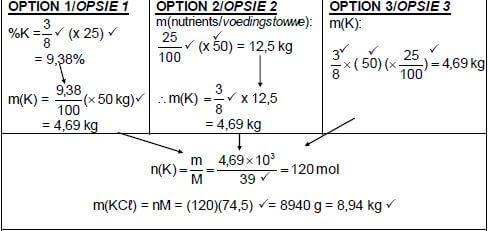 | ||
OPTION 4 m(K) = 9,38 x 50 = 4,69 kg m(100% KCℓ) = 4,69 x 100 |
[14]
TOTAL: 150