PHYSICAL SCIENCES GRADE 12 PAPER 2 MEMORANDUM - NSC PAST PAPERS AND MEMOS SEPTEMBER 2017
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES
GRADE 12
PAPER 2
NSC PAST PAPERS AND MEMOS
SEPTEMBER 2017
GUIDELINES FOR MARKING
This section provides guidelines for the way in which marks will be allocated. The broad principles must be adhered to in the marking of Physical Sciences tests and examinations.
1.1 MARK ALLOCATION
1.1.1 Definitions/Definisies: Two marks will be awarded for a correct definition. No marks will be awarded for an incorrect or partially correct definition.
1.1.2 Calculations:
- Marks will awarded for: correct formula, correct substitution, correct answer with unit.
- No marks will be awarded if an incorrect or inappropriate formula is used, even though there may be relevant symbols and applicable substitutions.
1.1.3 Explanations and interpretations:
Allocation of marks to questions requiring interpretation or explanation e.g. AS 1.4, 2.2, 2.3, 3.1, 3.2 and 3.3, will differ and may include the use of rubrics, checklists, memoranda, etc. In all such answers emphasis must be placed on scientific concepts relating to the question.
1.2 FORMULAE AND SUBSTITUTIONS/FORMULES EN SUBSTITUSIE
1.2.1 Mathematical manipulations and change of subjects of appropriate formulae carry no marks, but if a candidate starts with the correct formula and then changes the subject of the formula incorrectly, marks will be awarded for the formula and the correct substitutions. The mark for the incorrect numerical answer is forfeited.
1.2.2 When an error is made during substitution into a correct formula, a mark will be awarded for the correct formula and for the correct substitutions, but no further marks will be given.
1.2.3 Marks are only awarded for a formula if a calculation had been attempted, i.e. substitutions have been made or a numerical answer given.
1.2.4 Marks can only be allocated for substitutions when values are substituted into formulae and not when listed before a calculation starts.
1.2.5 All calculations, when not specified in the question, must be done to two decimal places.
1.3 UNITS
1.3.1 Candidates will only be penalised once for the repeated use of an incorrect unit within a question or sub-question.
1.3.2 Units are only required in the final answer to a calculation.
1.3.3 Marks are only awarded for an answer, and not for a unit per se. Candidates will therefore forfeit the mark allocated for the answer in each of the following situations:
- correct answer + wrong unit
- wrong answer + correct unit
- correct answer + no unit.
1.3.4 SI units must be used except in certain cases, e.g. V.m-1 instead of N.C-1, and cm.s-1 or km.h-1instead of m.s-1 where the question warrants this. (This instruction only applies to Paper 1).
1.4 POSTIVE MARKING
Positive marking regarding calculations will be followed in the following cases:
1.4.1 Sub-question to sub-question: When a certain variable is calculated in one sub-question (e.g. 3.1) and needs to be substituted in another (3.2 or 3.3), e.g. if the answer for 3.1 is incorrect and is substituted correctly in 3.2 or 3.3, full marks are to be awarded for the subsequent sub questions.
1.4.2 A multi-step question in a sub-question: If the candidate has to calculate, for example, current in the first step and gets it wrong due to a substitution error, the mark for the substitution and the final answer will be forfeited.
1.4.3 If a final answer to a calculation is correct, full marks will not automatically be awarded. Markers will always ensure that the correct/ appropriate formula is used and that workings, including substitutions, are correct.
1.4.4 Questions where a series of calculations have to be made (e.g. a circuit diagram question) do not necessarily always have to follow the same order. FULL MARKS will be awarded provided it is a valid solution to the problem. However, any calculation that will not bring the candidate closer to the answer than the original data, will not count any marks.
1.4.5 If one answer or calculation is required, but two given by the candidate, only the first one will be marked, irrespective of which one is correct. If two answers are required, only the first two will be marked, etc.
1.4.6 Normally, if based on a conceptual mistake, an incorrect answer cannot be correctly motivated. If the candidate is therefore required to motivate in question 3.2 the answer given to question 3.1, and 3.1 is incorrect, no marks can be awarded for question 3.2. However, if the answer for e.g. 3.1 is based on a calculation, the motivation for the incorrect answer for 3.2 could be considered.
1.4.7 If instructions regarding method of answering are not followed, e.g. the candidate does a calculation when the instruction was to solve by construction and measurement, a candidate may forfeit all the marks for the specific question.
1.4.8 For an error of principle, no marks are awarded (Rule 1) e.g. If the potential difference is 200 V and resistance is 25 Ω, calculate the current.
CORRECT | ANSWER (1) | POSSIBLE | ANSWER (2) | POSSIBLE |
I =V | R =V | R =V x | R =V | I =V |
1.5 GENERAL PRINCIPLES OF MARKING IN CHEMISTRY
The following are a number of guidelines that specifically apply to Paper 2.
1.5.1 When a chemical FORMULA is asked, and the NAME is given as answer, only one of the two marks will be awarded. The same rule applies when the NAME is asked and the FORMULA is given.
1.5.2 When redox half-reactions are to be written, the correct arrow should be used. If the equation is the correct answer, the following marks will be given:
H2S → S + 2H+ + 2e-(2/2)
- H2S ⇋ S + 2H+ + 2e-(1/2)
- H2S ← S + 2H+ + 2e-(0/2)
- S + 2H+ + 2e- ← H2S (2/2)
- S + 2H+ + 2e- ⇋ H2S (0/2)
1.5.3 When candidates are required to give an explanation involving the relative strength of oxidising and reducing agents, the following is unacceptable:
- Stating the position of a substance on Table 4 only (e.g. Cu is above Mg).
- Using relative reactivity only (e.g. Mg is more reactive than Cu).
- The correct answer would for instance be: Mg is a stronger reducing agent than Cu, and therefore Mg will be able to reduce Cu2+ ions to Cu. The answer can also be given in terms of the relative strength as electron acceptors and donors.
1.5.4 One mark will be forfeited when the charge of an ion is omitted per equation.
1.5.5 The error carrying principle does not apply to chemical equations or half-reactions. For example, if a learner writes the wrong oxidation/reduction half-reaction in the sub-question and carries the answer to another sub-question (balancing of equations or calculations of Eθcell) then the learner is not credited for this substitution.
1.5.6 When a calculation of the cell potential of a galvanic cell is expected, marks will only be awarded for the formula if one of the formulae indicated on the data sheet (Table 2) is used. The use of any other formula using abbreviations etc. will carry no marks.
1.5.7 In the structural formula of an organic molecule all hydrogen atoms must be shown. Marks will be deducted if hydrogen atoms are omitted.
1.5.8 When a structural formula is asked, marks will be deducted if the candidate writes the condensed formula.
1.5.9 When an IUPAC name is asked, and the candidate omits the hyphen (e.g. instead of 1-pentene the candidate writes 1 pentene), marks will be forfeited.
MEMORANDUM
QUESTION 1
1.1 B ✔✔ (2)
1.2 D ✔✔ (2)
1.3 A ✔✔ (2)
1.4 C ✔✔ (2)
1.5 C ✔✔ (2)
1.6 C ✔✔ (2)
1.7 A ✔✔ (2)
1.8 B ✔✔ (2)
1.9 C ✔✔ (2)
1.10 C ✔✔ (2) [20]
QUESTION 2
2.1.1 B ✔ (1)
2.1.2 F ✔ (1)
2.2.1 pentan-2-one ✔✔
Accept : 2-pentanone
IF/AS pentanone one mark (2)
2.2.2 3-bromo-3,4-dimethylhexane
Marking criteria:
- Correct stem i.e.hexane ✔
- First substituent, bromo, correctly identified ✔
- Second substituent, dimethyl, correctly identified ✔
- Subtract a mark for missing hyphens, commas ,incorrect numbering. (3)
2.3.1 Ester ✔ (1)
2.3.2 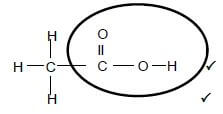 (2)
(2)
Marking criteria:
- Functional group correct. 1/2
- Whole structure correct. 2/2
2.3.3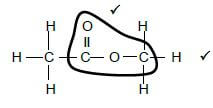 (2)
(2)
Marking criteria:
- Functional group correct. 1/2
- Whole structure correct. 2/2
2.4.1 Organic molecules with the same molecular formula but different structural formulae. (2)
2.4.2 Ethyl ✔ methanoate (2)
2.5.1 Thermal (1)
2.5.2 C4H10 ✔ (1) [16]
QUESTION 3
3.1 Secondary ✔ (1)
3.2
3.2.1 Substitution ✔ (1)
3.2.2 Elimination ✔/dehydrohalogenation (1)
3.2.3 Addition ✔/hydration (1)
3.3 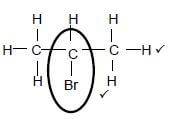 (2)
(2)
Marking criteria:
- Functional group correct 1/2
- Whole structure correct. 2/2
3.4 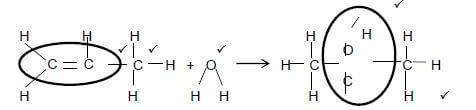 (5)
(5)
Accept H2O
3.5 Concentrated ✔ (1) [12]
QUESTION 4
4.1.1 The pressure exerted by a vapour at equilibrium with its liquid in a closed system.✔✔ (2 OR0) (2)
4.1.2 Molecular mass (size) ✔ (1)
4.1.3 E has two sides for hydrogen bonding and F has one OR E forms dimers ✔ (1)
4.2.1 From A to C/Van A na C
- Chain length decreases/surface area decreases/More branches. ✔
- Strength of intermolecular forces/London forces/dispersion forces/induced dipole forces decreases. ✔
- Less energy needed to overcome/break intermolecular forces. ✔
OR
From C to A/Van C na A
- Chain length increase/surface area increases/Less branches. ✔
- Strength of intermolecular forces /London forces/dispersion forces/induced dipole forces increases✔
- Less energy needed to overcome/break intermolecular forces increases. ✔ (3)
4.2.2 Surface area ✔ /Position of side (methyl) chain (1)
4.2.3 n(O2) = V/Vm
= 96/24 ✔
= 4 mol
n(C6H14) = 2/19 x 4 ✔
= 0,42 mol
Marking Criteria:
- Divide by 22,4✔
- Use ratio ✔
- Multiply by 4163✔
- Final answer✔
Net energy released = 0,42 x - 4163 = -1 748 kJ/mol ✔
NOTE: Accept positive answer (4) [12]
QUESTION 5
5.1.1 (Use zinc) powder ✔ / Increase surface area (of zinc) (1)
5.1.2 Increase (temperature) ✔ / Heat (1)
5.2.1 Reaction is complete✔ / Reaction stops/Zinc is used up/ (1)
5.2.2 t1✔
Gradient highest✔ / Steepest gradient (2)
5.3.1 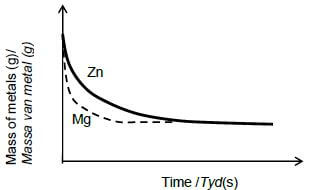
Criteria for graph Mg
- Shape as shown with a steeper gradient below graph of Zn, starting from the same point and ends at the same point.✔
- Graph becomes horizontal in less time ✔ (2)
5.3.2 Cu2+ is a stronger oxidising agent than H+ ✔✔ OR H+is a weaker oxidising agent than Cu2+ (2)
5.4.1 Decrease in temperature ✔ (1)
5.4.2
- Increase in temperature increases reaction rate. ✔
- Kinetic energy of particles increases as temperature increases. ✔
- More particles will have sufficient/enough (kinetic) energy/ Ek ≥ EA. ✔
- More effective collisions per unit time/second. ✔
OR
- Decrease in temperature decreases reaction rate ✔
- Kinetic energy of particles decreases as temperature decreases ✔
- Less particles will have sufficient/enough (kinetic) energy/Ek ≥ EA. ✔
- Less effective collisions per unit time/second. ✔ (4) [14]
QUESTION 6
6.1 A reaction is reversible when products can be converted back to reactants.✔✔. (2 or/of 0) (2)
6.2.1 Decreases ✔ (1)
6.2.2 Increases ✔ (1)
6.3 10-3 ✔ (1)
6.4 Marking Criteria:
- Equilibrium n(AX2) = Equilibrium c(AX2) x V ✔
- Change in n(AX2) = equilibrium n(AX2) – initial n(NH3) ✔
- USE RATIO for change in n(AX2) and change in n(X2) change.✔
- n(equilibrium) = n(initial) – n (change) for n(X2) ✔
- Divide equilibrium n(X2) by V to calculate equilibrium c(X2).✔
- Correct Kc expression (formulae in square brackets).✔
- Substitution of concentrations into Kc expression. ✔
- Final answer/Finale antwoord: 1,84 dm-3 ✔
POSITIVE MARKING from QUESTION 6.3
OPTION 1
3X2 | 2AX2 | |
ninitial(mol) | 0,46 | 0 |
nchange(mol) | 0,15 V | + 0,1 V ✔ |
nequilibrium(mol) | 0,46 – 0,15 V ✔ | 0,1 V ✔ |
cequilibrium(mol.dm-3) | 0,46 – 0,15 ✔ | 0,1 |
- Ratio ✔
Kc = [AX2]2 / [X2]3✔
10-3 = (0,1)2/ (0,46 / V – 0,15)3 ✔
V = 0,2 dm-3✔
OPTION 2
Kc = [AX2]2 / [X2]3 ✔
10-3 = (0,1)2/ [X2]3
[X2] = 2,15 mol.dm-3
3X2 | 2AX2 | |
ninitial(mol) | 2,15 V + 0,15 V= 0,46 | 0 |
nchange(mol) | 0,15 V ✔ | 0,1 V ✔ |
nequilibrium(mol) | 2,15 V ✔ | 0,1V ✔ |
cequilibrium(mol.dm-3) ) | 2,15 | 0,1 |
2,15 V + 0,15 V = 0,46 ✔
V = 0,2 dm3 ✔
- Ratio✔
OPTION 3
USING CONCENTRATIONS
- Kc = [AX2]2 / [X2]3 ✔
10-3 = (0,1)2/ [X2]3
[X2] = 2,15 mol.dm-3
3X2 | 2AX2 | |
cinitial | 2,15 + 0,15 = 2,3 | 0 |
cchange | 0,15 ✔ | 0,1✔ |
cequilibrium | 2,15 | 0,1✔ |
- Ratio✔
c = n/V ✔
2,3 = 0,46 /V ✔
V = 0,2 dm-3 ✔ (8)
6.5 Decreases✔
- An increase in temperature causes a decrease in Kc. ✔
- When the temperature is increased, the reaction that will oppose this increase in temperature, will be favoured. ✔
- Reverse reaction is favoured by a decrease in temperature. ✔
OR
- The forward reaction is exothermic. ✔
- An increase in temperature favours the endothermic reaction. ✔
- The reverse reaction is favoured. ✔ (4) [18]
QUESTION 7
7.1.1 Hydrolysis ✔ (1)
7.1.2 Weak ✔
- Kb value is < 1 ✔
OR
- Kb value is low (2)
7.1.3 Acids ✔
- Both act as proton (H+)donors ✔ /Donate protons(H+)/Lose protons(H+). (2)
7.2.1 Burette ✔(1)
7.2.2 20 cm3 ✔ (1)
7.2.3 POSITIVE MARKING from QUESTION 7.2.2/
- n = Cv ✔
n(NaOH) = 0,2 x 20/1000 ✔= 0,004 mol ✔ (4 x 10-3 mol) (3)
7.2.4 POSITIVE MARKING FROM QUESTION 7.2.3
- n(H2) = ½ (0,004)✔ = 0,002 mol (2 x 10-3 mol)
c(H2X)= n / V = 0,002/ (40/1000) ✔= 0,05 mol.dm-3
pH = - log[H3O+] ✔= - log(2 x 0,05) ✔= 1 ✔(5)
Marking Criteria :
- Use of ratio NaOH : H2X ✔
- Substitution into c = n/V to calculate n(H2X).
- Use formule: pH = -log [H3O+] ✔
- Substitution of 2 x c(H2X) into [H3O+]✔
- Final answer✔
7.2.5 Neutral ✔
- (Titration of) strong base with a strong acid. ✔ (2) [18]
QUESTION 8
8.1 It is solution/liquid/dissolved substance that conducts electricity through the movement of ions. ✔. (2)
8.2 Positive ✔
- It is the cathode. ✔
OR
- It is the electrode where reduction takes place. (2)
8.3.1 1 mol.dm-3 ✔ (1)
8.3.2 ✔ ✔ ✔
Ti (s)/ Ti3+(aq) // Ag+ (aq)/ Ag(s) Accept Ti/ Ti3+// Ag+/ Ag (3)
8.4 E0cell = E0cathode – E0anode ✔
2,43 = 0,80 – E0anode
E0anode = -1,63 V ✔
Notes:
- Accept any other correct formula from the data sheet.
- Any other formula using unconventional abbreviations, e.g. E0cell = E0OA – E0RA 3/4 (4)
8.5 3 Mg + 2 Ti3+ ✔ ⇌ 3 Mg2+ + 2 Ti ✔ ✔ balancing
Reactants ✔ Products ✔ Balancing ✔
Accept single arrow (3) [17]
QUESTION 9
9.1 Negative ✔
9.2 To improve (electrical) conductivity. (1)
9.3 Decreases ✔
- Cu Cu2++ 2e- ✔✔
Ignore phases (3)
9.4 n(Cu) = ½ x 2✔ Cu2++ 2e- → Cu
= 1 mol
m(Cu) = nM = 1(63,5) ✔= 63,5 g
% Purity = m(pure)/ m(impure) x 100
m(impure) = 63,5 x 100 ✔
95,7 ✔
= 66,35 g ✔ (5) [10]
QUESTION 10
10.1.1 Ammonia ✔(1)
10.1.2 Ostwald(process) ✔ (1)
10.1.3 NO ✔ (1)
10.1.4 HNO3 ✔ and NO ✔ (2)
10.1.4 NH3(g) + HNO3(aq) ✔ NH4NO3 ✔ Bal. ✔ (3)
Notes:
- Reactants ✔ Products ✔ Balancing ✔
- Ignore ⇌ and phases
- Marking rule 6.3.10
10.2.1 The percentage of fertiliser in the bag ✔/ The percentage of the primary nutrients (N:P:K) in the bag of fertilizers. (1)
10.2.2 Dead zones ✔ (1)
10.2.3 % N in (NH4)2SO4 = 2(14) x 100 ✔ = 21,21%
132
m(N) = 21,21 x 39,6 ✔ = 8,399 kg
100
% N in bag = 7/15 x 36 ✔= 16,8%
16,8 x m = 8,399 kg ✔
100
m = 50 kg ✔ (5) [15]
Marking criteria:
- Calculate % N in (NH4)2SO4 ✔
- Calculate m(N) in (NH4)2SO4 ✔
- % N in bag of fertiliser✔
- Calculate mass m ✔
- Final answer: 50 kg ✔
TOTAL 150