PHYSICAL SCIENCES: CHEMISTRY PAPER 2 GRADE 12 QUESTIONS - NSC PAST PAPERS AND MEMOS FEBRUARY/MARCH 2018
Share via Whatsapp Join our WhatsApp Group Join our Telegram GroupPHYSICAL SCIENCES: CHEMISTRY
PAPER 2
GRADE 12
NSC PAST PAPERS AND MEMOS
FEBRUARY/MARCH 2018
INSTRUCTIONS AND INFORMATION
- Write your centre number and examination number in the appropriate spaces on the ANSWER BOOK.
- This question paper consists of TEN questions. Answer QUESTION 5.3 on the attached GRAPH PAPER. Answer ALL the questions in the ANSWER BOOK.
- Start EACH question on a NEW page in the ANSWER BOOK.
- Number the answers correctly according to the numbering system used in this question paper.
- Leave ONE line between two subquestions, for example between QUESTION 2.1 and QUESTION 2.2.
- You may use a non-programmable calculator.
- You may use appropriate mathematical instruments.
- You are advised to use the attached DATA SHEETS.
- Show ALL formulae and substitutions in ALL calculations.
- Round off your FINAL numerical answers to a minimum of TWO decimal places.
- Give brief motivations, discussions, et cetera where required.
- Write neatly and legibly.
QUESTIONS
QUESTION 1: MULTIPLE-CHOICE QUESTIONS
Various options are provided as possible answers to the following questions. Choose the answer and write only the letter (A-D) next to the question number (1.1-1.10) in the ANSWER BOOK, for example 1.11 D.
1.1 Which ONE of the following is the general formula of alkynes?
- CnH2n
- C2nH2n
- CnH2n - 2
- CnH2n + 2 (2)
1.2 The type of reaction that takes place when a carboxylic acid and an alcohol react in the presence of an acid:
- Addition
- Hydrolysis
- Substitution
- Esterification (2)
1.3 Which ONE of the following isomers has the LOWEST boiling point?
- CH3CH2CH2CH2CH2CH3
- CH3CH2C(CH3)2CH3
- CH3CH(CH3)CH2CH2CH3
- CH3CH2CH(CH3)CH2CH3 (2)
1.4 Which ONE of the reaction rate versus time graphs below best represents the reaction between magnesium and EXCESS dilute hydrochloric acid? 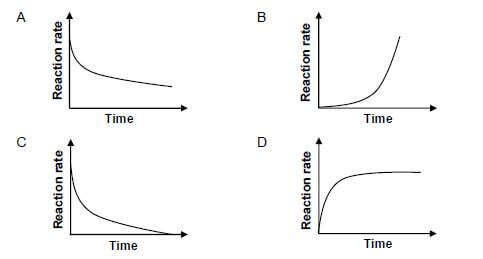 (2)
(2)
1.5 Which ONE of the following will NOT affect the equilibrium position of reversible chemical reactions?
- Temperature
- Catalyst
- Pressure
- Concentration (2)
1.6 The following equilibrium exists in pure water at 25 °C.
2H2O(ℓ) ⇌ H3O+(aq) + OH(aq) ∆H > 0
At this temperature, the pH = 7 and Kw = 1 x 10-14. The temperature of the water is now increased to 90 °C. Which ONE of the following is TRUE at the new temperature?
- pH = 7
- [H3O+] = [OH ]
- [H3O+][OH ] = 10-14
- [H3O+] = 10-7 mol∙dm-3 (2)
1.7 A hydrochloric acid solution is titrated against an ammonia solution. The balanced equation for the reaction is:
HCℓ(aq) + NH4OH(aq) → NH4Cℓ(aq) + H2O(ℓ)
Which ONE of the following gives the pH of the solution at the end point and the reason for this pH? (2)
| pH | REASON | |
| A | 3 | H3O+(aq) is formed during the ionisation of HCℓ(aq). |
| B | 5 | H3O+(aq) is formed during hydrolysis of NH (aq). |
| C | 7 | Neutralisation takes place at the end point. |
| D | 8 | OH-(aq) is formed during hydrolysis of NH4 (aq). |
1.8 A decrease in the oxidation number of an atom during a chemical reaction is known as …
- redox.
- oxidation.
- reduction.
- electrolysis. (2)
1.9 The two half-reactions below are used to construct a galvanic cell.
X+(aq) + e- ⇌ X(s) Eθreduction = + 0,15 V
Y2+(aq) + 2e- ⇌ Y(s) Eθreduction = - 0,15 V
Which ONE of the statements below is CORRECT when the cell is in operation?
- X+(aq) is reduced.
- Y(s) is reduced.
- X(s) | X+(aq) is the negative electrode.
- Electrons flow from X(s) to Y(s) in the external circuit. (2)
1.10 Which ONE of the following is CORRECT for the industrial preparation of sulphuric acid? (2)[20]
| PROCESS | CATALYST | |
| A | Ostwald | Platinum |
| B | Haber | Iron |
| C | Contact | Iron |
| D | Contact | Vanadium pentoxide |
QUESTION 2 (Start on a new page.)
The letters A to E in the table below represent six organic compounds.
2.1 Write down the LETTER that represents EACH of the following:
2.1.1 A tertiary alcohol (1)
2.1.2 An aldehyde (1)
2.1.3 A ketone (1)
2.1.4 A functional isomer of compound B (1)
2.2 Write down the IUPAC name of:
2.2.1 Compound B (1)
2.2.2 Compound E (4)
2.3 Define positional isomers. (2)
2.4 Write down the STRUCTURAL FORMULA of:
2.4.1A positional isomer of compound C (2)
2.4.2 Compound D (2)
2.4.3The organic acid that will react with compound C to form butyl propanoate (2) [17]
QUESTION 3 (Start on a new page.)
Study the vapour pressure versus temperature graphs for three organic compounds, X, Y and Z, below which belong to different homologous series.
Atmospheric pressure is 100 kPa.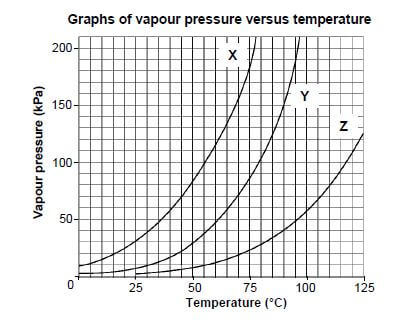
3.1 Write down the vapour pressure of compound Y at 90 °C. (1)
3.2 The graphs can be used to determine the boiling points of the three compounds.
3.2.1Define boiling point. (2)
3.2.2 Determine the boiling point of compound X. (1)
3.3 The homologous series to which the three compounds of similar molecular masses belong, were identified in random order as:
alcohol; carboxylic acid; ketone
3.3.1 Which compound (X, Y or Z) is the carboxylic acid? (1)
3.3.2 Explain the answer to QUESTION 3.3.1 by referring to the type of intermolecular forces in compounds of each of the homologous series above. (4)
3.3.3Compound X has three carbon atoms per molecule. Write down the IUPAC name of compound X. (1) [10]
QUESTION 4 (Start on a new page.)
Consider the incomplete equations for reactions I to IV below. P, Q, R and S are organic compounds.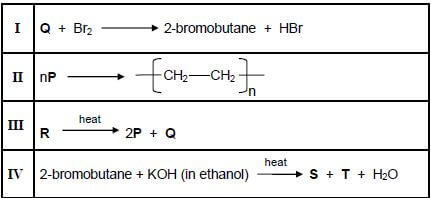
4.1Define a cracking reaction. (2)
4.2 Write down the reaction number (I, II, III or IV) that represents EACH of the following:
4.2.1A cracking reaction (1)
4.2.2An addition reaction (1)
4.2.3 A substitution reaction (1)
4.3Write down:
4.3.1ONE reaction condition for reaction I (1)
4.3.2 The compound (P, Q, R or S) that represents an unsaturated hydrocarbon (1)
4.3.3 The IUPAC name of compound P (1)
4.3.4 The molecular formula of compound R (2)
4.3.5The structural formula of compound Q (2)
4.3.6The structural formula of compound S (2) [14]
QUESTION 5 (Start on a new page.)
ANSWER QUESTION 5.3 ON THE ATTACHED GRAPH PAPER.
Learners use the reaction between sodium thiosulphate and hydrochloric acid to investigate one of the factors that affects reaction rate. The balanced equation for the reaction is:
Na2S2O3(aq) + 2HCℓ(aq) → 2NaCℓ(aq) + H2O(ℓ) + SO2(g) + S(s)
The diagram below shows the experimental setup.
In the first experiment, 50 cm3 of the sodium thiosulphate solution is added to 100 cm3 of a 2 mol∙dm-3 dilute hydrochloric acid solution in a flask that is placed over a cross drawn on a sheet of white paper. The hydrochloric acid is in EXCESS.
The time taken for the cross to become invisible, when viewed from the top, is recorded.
The experiment is then repeated four times with different volumes of the sodium thiosulphate solution. The results obtained are shown in the table below.
EXPERIMENT | VOLUME OF | VOLUME OF | TIME (s) | AVERAGE RATE |
1 | 50 | 0 | 22,7 | 4,4 |
2 | 40 | 10 | 28,6 | 3,5 |
3 | 30 | 20 | 38,5 | 2,6 |
4 | 20 | 30 | 58,8 | 1,7 |
5 | 10 | 40 | 111,1 | 0,9 |
5.1 Define reaction rate. (2)
5.2 How does the concentration of the sodium thiosulphate solution used in experiment 2 compare to that used in experiment 5? Choose from MORE THAN, LESS THAN or EQUAL TO. (1)
5.3 Draw a graph of average reaction rate versus volume of sodium thiosulphate used on the attached GRAPH SHEET.
(ATTACH THIS GRAPH SHEET TO YOUR ANSWER BOOK.) (3)
5.4 Use the information in the graph to answer the following questions.
5.4.1Determine the volume of dilute sodium thiosulphate solution that needs to react in order for the cross to become invisible in 40 seconds.
USE DOTTED LINES ON THE GRAPH TO SHOW HOW YOU ARRIVED AT THE ANSWER. (3)
5.4.2Write down a conclusion for this investigation. (2)
5.5 Use the collision theory to explain the effect of an increase in concentration on reaction rate. (3)
5.6 The mass of sulphur produced in experiment 1 is 1,62 g. Calculate the mass of the sodium thiosulphate used in experiment 1. (4) [18]
QUESTION 6 (Start on a new page.)
6.1 A reversible gaseous reaction is allowed to reach equilibrium in a closed container at different temperatures and pressures.
The graph below shows the percentage yield for this reaction at 30 kPa as the temperature is increased.
Use the information in the graph above to answer the following questions.
6.1.1 State Le Chatelier's principle. (2)
6.1.2 The heat of reaction (∆H) for the forward reaction is POSITIVE. Use Le Chatelier's principle to explain this statement. (3)
The graph below show the percentage yield for this reaction as pressure changes at constant temperature.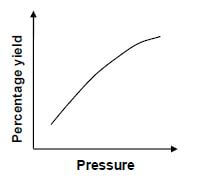
6.1.3 Explain the effect of an increase in pressure on the equilibrium position of a reaction. (2)
6.1.4 Which ONE of the following equations (I, II or III) represents the equilibrium above?
- 2A(g) + 3B(g) ⇌ 3C(g)
- A(g) + B(g) ⇌ 3C(g)
- A(g) + B(g) ⇌ 2C(g) (2)
6.2 A mixture of 0,2 moles of hydrogen chloride (HCℓ) and 0,11 moles of oxygen gas (O2) is sealed in a 200 cm3 flask at a certain temperature.
The reaction reaches equilibrium according to the balanced equation below:
4HCℓ(g) + O2(g) ⇌ 2Cℓ2(g) + 2H2O(g)
It is found that 1,825 g of hydrogen chloride is present at equilibrium.
Calculate the equilibrium constant, Kc, for this reaction at this temperature. (9) [18]
QUESTION 7 (Start on a new page.)
7.1 The balanced equation below represents the first step in the ionisation of sulphuric acid (H2SO4) in water:
H2SO4(ℓ) + H2O(ℓ) ⇌ H3O+(aq) + HSO-4 (aq)
7.1.1 Write down the FORMULAE of the TWO bases in the equation above. (2)
7.1.2 Is sulphuric acid a STRONG or a WEAK acid? Give a reason for the answer. (2)
7.2 Learners use the reaction of a 0,15 mol∙dm-3 sulphuric acid solution with a sodium hydroxide solution in two different experiments. The balanced equation for the reaction is:
H2SO4(aq) + 2NaOH(aq) → Na2SO4(aq) + H2O(ℓ)
7.2.1 They use 24 cm3 of H2SO4(aq) in a titration to neutralise 26 cm3 of NaOH(aq).
Calculate the concentration of the NaOH(aq). (5)
7.2.2 In another experiment, 30 cm3 of the H2SO4(aq) is added to 20 cm3 of a 0,28 mol∙dm-3 NaOH solution in a beaker.
Calculate the pH of the final solution. (8) [17]
QUESTION 8 (Start on a new page.)
8.1 A group of learners use the redox reaction below to construct an electrochemical cell.
Sn2+(aq) + 2Ag+(aq) → 2Ag(s) + Sn4+(aq)
8.1.1 Define a reducing agent in terms of electron transfer. (2)
8.1.2 Name a substance that should be used as electrode in the anode half-cell. (1)
8.1.3 Write down the NAME or FORMULA of the reducing agent. (1)
8.1.4 Write down the cell notation of the cell. (3)
8.1.5 Calculate the initial emf of this cell under standard conditions. (4)
8.2 In a separate experiment, the learners place magnesium ribbon in a beaker containing a blue solution of copper(II) sulphate. After a while the solution becomes colourless.
8.2.1 State ONE observable change in the beaker, besides a colour change of the solution, that the learners can make. (1)
8.2.2 Refer to the relative strengths of oxidising agents or reducing agents to explain why the solution becomes colourless. (3) [15]
QUESTION 9 (Start on a new page.)
The graph below represents the changes in mass that occur at electrode A and electrode B in an electrolytic cell during the purification of copper.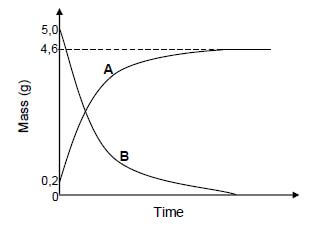
9.1 Define electrolysis. (2)
9.2 Which graph, A or B, represents the change in mass of the anode during electrolysis? (1)
9.3 Write down the equation of the half-reaction which takes place at the cathode of this cell.(2)
9.4 Use the information in the graph and calculate the percentage purity of the impure copper. (4) [9]
QUESTION 10 (Start on a new page.)
10.1 The diagram below shows processes involved in the production of fertiliser X and fertiliser Z.
Write down the:
10.1.1 Balanced equation for the formation of product Q (3)
10.1.2 FORMULA of fertiliser X (1)
10.1.3 NAME of process A (1)
10.1.4 NAME of fertiliser Z (1)
10.2 A 10 kg bag of NPK fertiliser is labelled 6 : 1 : 5 (22).
10.2.1 What is the meaning of NPK? (1)
10.2.2 What is the meaning of (22) on the label? (1)
10.2.3 Calculate the mass of potassium in the bag. (4) [12]
TOTAL: 150
DATA FOR PHYSICAL SCIENCES GRADE 12 PAPER 2 (CHEMISTRY)
TABLE 1: PHYSICAL CONSTANTS
| NAME | SYMBOL | VALUE |
| Standard pressure | pθ | 1,013 x 105 Pa |
| Molar gas volume at STP | Vm | 22,4 dm3∙mol-1 |
| Standard temperature | Tθ | Tθ 273 K |
| Charge on electron | e | e -1,6 x 10-19 C |
| Avogadro’s constant | NA | NA 6,02 x 1023 mol-1 |
TABLE 2: FORMULAE
n = m | n = N NA |
| c = n V or c = m MV | n = V Vm |
| caVa= na cbVb nb | pH= -log[H3O+] |
| Kw = [H3O+][OH-] = 1x10-14 at 298K | |
| Eθ cell = Eθ cathode – Eθ anode Eθ cell = Eθ reduction – Eθ oxidation Eθ cell = Eθ oxidising agent – Eθ reducing agent |